Is the stem cell the superhero of medicine, come to heal all wounds and reverse aging?

Creative Commons Attribution- Share Alike 4.0 International by Author Vegas Bleeds Neon Modified by FRacco
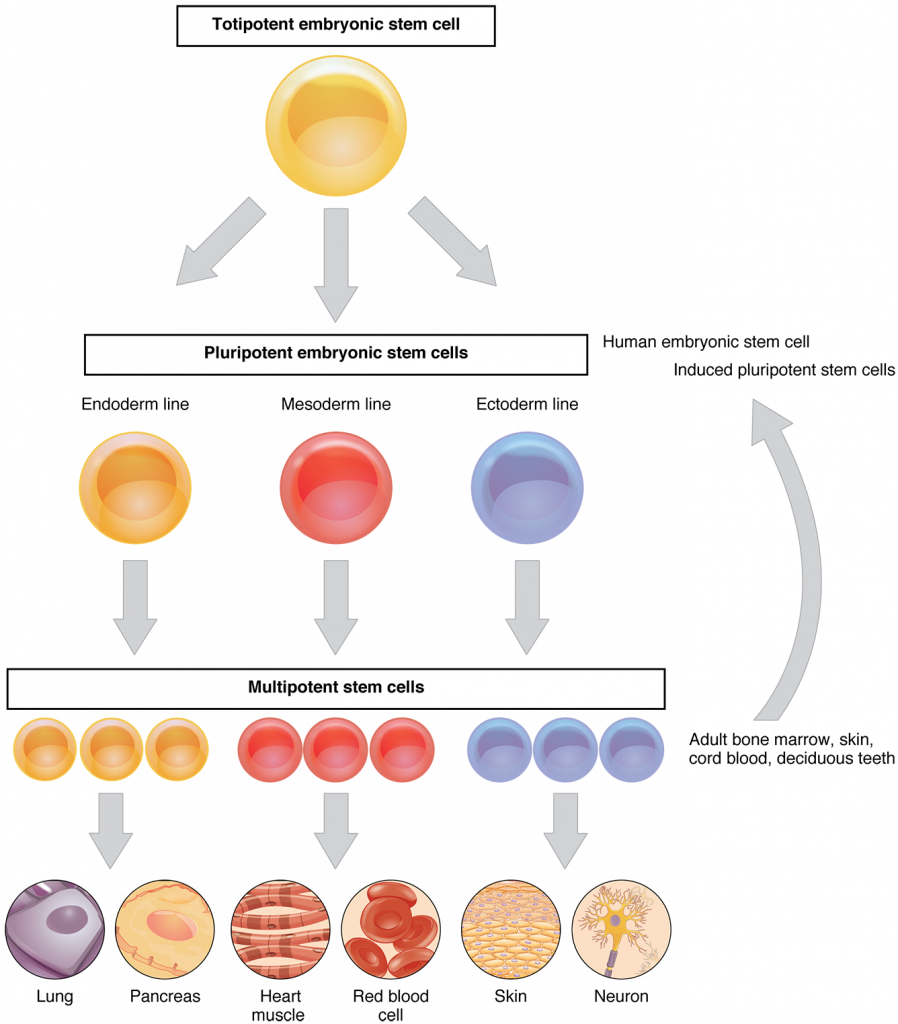
There has been a lot of hype in the public and academic worlds over the past 30-40 years regarding what these cells can do, and for good reason. These undifferentiated cells certainly have the potential to renew and grow any tissue type, and could possibly be used to grow organs. Stem cells can even be produced from differentiated cells in the body where they are then termed induced pluripotent stem cells (iPS), thus avoiding ethical issues regarding harvesting from embryos.
Of course, science quickly caught wind of this potential, although policy surrounding the research created barriers. First, in 1981 Martin Evans found embryonic stem (ES) cells in mice, then in 1998 human ES cells were cultured, and in 2006 Shinya Yamanaka discovered how to revert cells back to their pluripotent state, developing iPS cells. Between 2008-2012 stem cell papers saw 50% more citations than other papers in similar fields with much of this science focusing on regenerative medicine, with topics in hematopoietic stem cells, cancer, drug development, and more. Last year the Canadian Stem Cell Foundation received news that they would receive new government funding to push forward the sector, which is expected to have both therapeutic and economic success. In the US, on the other hand, restrictions on funding for stem cell research placed by Bush were lifted in 2009 by Obama, and concerns have been raised that Bush era regulations might return under Trump.
What do we know about these biological hero’s?
Lets get a handle on the basics of stem cell biology. Stem cells have three main properties: they continually divide and renew, are undifferentiated, and can differentiate to produce new cell types. In early embryonic development, differentiation occurs to produce the various tissues in the body. Adult stem cells cannot self renew for as long as embryonic stem cells before they become differentiated, and are more restricted to renewing a specific tissue, posing a particular challenge in the lab. Differentiation is not only influenced by the age of the stem cell and the life stage of the organism, but also by its genomic expression, environment, and epigenetic factors. But why can’t they continue to renew, to keep up with the physiological damage that we accumulate as we get older? Why would we need transplants in the first place if we haven’t physically removed the stem cells through trauma? In essence, what is making these cells age?
Studies of stem cell systems indicate that a lack of homeostasis, disruption in the balance of renewal and death of cells, may age tissues. For example, disruption of lymphocyte production decreases the immune response, and blood diseases like myeloid leukemia rise in incidence as humans age.
Intrinsic factors are also implicated, which are those factors due to stem cell biology. Aging hematopoietic cells were found to have decreased expression of lymphoid lineage genes (T, B, natural killer cells), increased expression in myeloid lineage genes (megakaryocytes, erythrocytes, granulocytes, macrophages), and increased self renewal. In a separate study, increased self renewal was identified in hematopoietic stem cells to increase double and single strand DNA damage. The damage resulted from repeat induced physiological stress in mice, which caused the stem cells to leave their dormant state. The authors hypothesized that because this occurred repeatedly, the cells began to experience replicative stress, which could potentially later lead to total failure of a renewal system, causing diseases like bone marrow failure syndrome or Fanconi anaemia. As such, it is possible that other renewal systems in other animal species respond in the same way to repeat physiological stressors.
With this knowledge, and current policies, where do we stand with stem cell research?
The movement with iPS cells was halted, Dr. Shinya Yamanaka told New York Times in January of 2017, due to finding mutations in the cells of the first patient of their trial – a risk expected when increasing the proliferation and thus mutation rate. Different approaches are now being taken. Dr. Yamanaka also states that stem cells are not a single solution for all disease, due to the complex cell types involved in many diseases. He also raises concerns regarding the speed of the science as compared to the speed of ethical discussion.
Stem cells are both critical to our early development and to the maintenance of tissues throughout life. They even seem to have promise for treating several diseases with specific cell types failures. However, the initial hype likely came from the romantic idea of immortality inferred from the discovery of a self-renewing cell. As for the science, there is more to do with stem cell systems so that they can be used to treat diseases like Parkinson’s, retinal and corneal diseases, heart and liver failure, diabetes, spinal cord injuries, joint disorders, and some blood disorders, as Dr. Yamanaka stated. While this isn’t promising to make the old young again, it is still well worth the chase to help the amount of people affected by these diseases from single cell type dysfunction.

Recent Comments