Who hasn’t thought about living forever? Modern society has an obsession with increasing longevity and quality of life throughout the lifespan. We have all seen the advertisements in magazines and on social media trying to sell us juice cleanses and fad diets that will (allegedly) help us live longer and healthier lives. But what if the fountain of youth is not a fountain at all? What if the secret to longevity is something that is already an integral part to our daily routines

Runner on treadmill. By:E’Lisa Campbell, Permission: CC BY-SA 2.0. Not Modified.
Regular physical activity has been investigated time and time again as a method of improving longevity and quality of life as we age, and more evidence is accumulating as we speak. Physical activity is known to decrease the likelihood of developing age-related diseases including cardiovascular disease (CVD), cancer, and diabetes. Not to mention it guards against age-related cognitive decline and neurodegenerative diseases including Alzheimer’s disease. However, the cellular mechanisms responsible for these benefits are unknown, and are therefore, a hot topic for cell biology researchers.
We are all aware (and even frightened) of the obvious and unpleasant signs of aging such as aching joints on cold mornings and forgetting to pick up milk from the store more often than we would like to admit. However, we do not normally think about how the activity of our individual cells changes throughout our lifespan. Cell division is critical to maintain the integrity and the functioning of an organism. It is particularly important for cells exposed to the elements such as skin, gut and lung cells. These cells are damaged easily and can lose their ability to function. If our cells did not have the capacity to reproduce, we would not be able to outlive our individual cells. However, there is a small price to pay for this regenerative capacity.
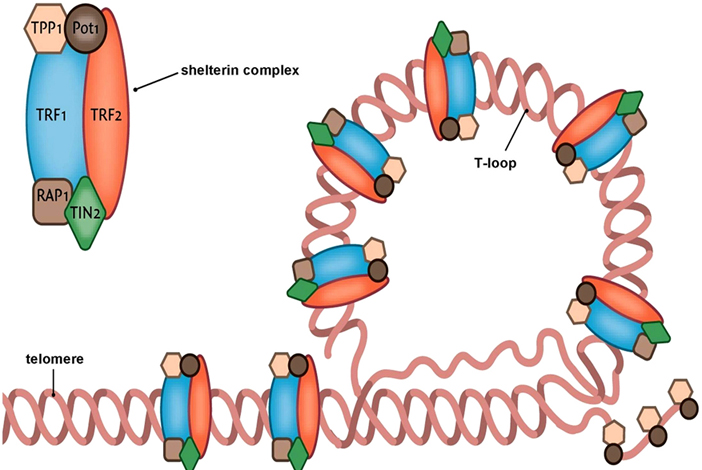
The shelterin-complexes on a telomere. By: Beyond the Dish, Permission: CC BY-SA 4.0. Not Modified.
Telomeres are specialized DNA structures that reside at the ends of our chromosomes that protect them from degradation. The theory that chromosome ends play a role in chromosome stability was proposed in the 1930s by McClintock and Muller. Through their experiments on maize and fruit flies, they both proposed that telomeres are structures that ensure chromosome stability, and faithful segregation. These finding have stood the test of time. It is now widely accepted that every time a cell divides, there is a loss of telomeric base pairs due to the inability of DNA polymerase to fully replicate the lagging strand. Therefore, telomere length can be an indicator of replicative history. The bad news is that this loss imposes a replicative limit. Shortening of telomeres leads to the eventual disruption of the protective protein complex called shelterin. This leads to cellular senescence, the progressive and irreversible loss of replicative capacity in somatic cells.
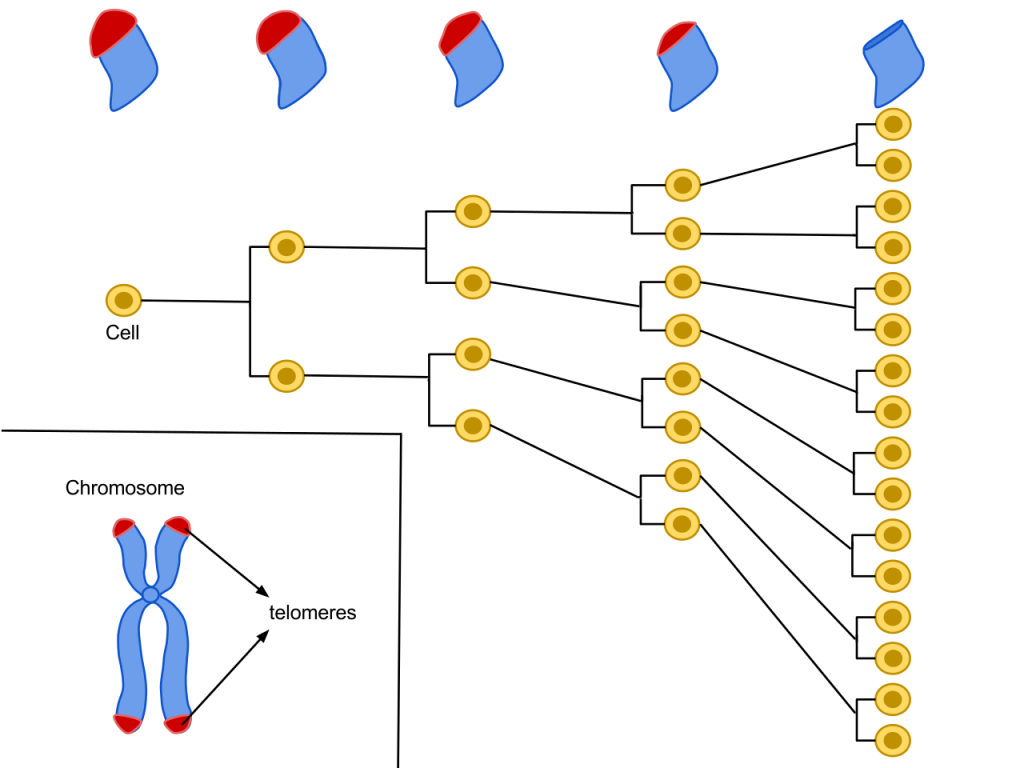
Progressive telomere shortening due to cell division. By: Azmistowski17, Permission: CC BY-SA 4.0. Not Modified.
Senescence is triggered when the terminal restriction fragment of the telomere reaches a critical length. This disrupts the shelterin complex and exposes and uncapped double-stranded chromosomal end. This signals a DNA damage response, which prevents cells from entering S-phase of the cell cycle. This process is not all bad. In fact, it is necessary for many important cellular functions including embryonic development and tumour suppression. However, it has been causally linked to age-related diseases such as CVD, diabetes and osteoarthritis. Moreover, senescence can produce significant changes to gene expression that impact cell functioning and morphology for the worst. Senescent cells can develop into a pro-inflammatory phenotype, which can initiate senescence in young cells and therefore, contribute to tissue dysfunction and even cancer.
Now that I have outlined the grim and inevitable reality of cellular ageing, I’ll move onto the good news. Scientists may have identified a method to slow down the process. There have been several studies that have demonstrated a relationship between regular physical activity and longer telomeres. A recent study published in Preventative Medicine found that physical activity was inversely related to telomere length in a large random sample of 5823 adults living in the United States. They observed that adults who reported high levels physical activity had a biological aging advantage of 9 years compared to sedentary participants. Significant differences between adults reporting high activity compared to participants reporting low or moderate activity were also observed even when taking into account lifestyle factors including smoking, body mass index, and alcohol consumption. A 2015 meta-analysis also demonstrated similar trends in other populations including post-menopausal women and professional athletes. Therefore, there is some evidence to suggest that physical activity is a mediating factor on telomere length. However, the cellular mechanisms that produce this effect remain unknown.
Even though the cellular mechanisms behind these benefits are still being investigated, there have been some potential breakthroughs. Telomeres are dynamic systems that are subject to microenvironment and enzyme activity. Some cell types like adult proliferating cells and immune cells, house a ribonucleioprotein called telomerase. Telomerase maintains and lengthens telomeres and therefore, starves off senescence. A 2009 study observed an increase is telomerase activity, expression of telomere stabilizing proteins and a decrease in cell-cycle inhibitors and apoptosis regulators like p53 in aortic leukocytes of mice after 3 weeks of voluntary running. However, there have been mixed results in human populations. In addition, researchers have suggested that longer telomeres are due to a reduction in oxidative stress due to anti-inflammatory processes up-regulated by exercise, as oxidative-stress has been associated with reduced telomere length. However, this was only speculated. For now, researchers are still falling down the immunological rabbit hole to understand how inflammatory processes influence telomere biology.
There is still a lot to learn about how exercise mediates telomere length and stability. However, there is a plethora of robust studies that have corroborated the effect of exercise on telomere length. The bad news is that finally using that gym membership of yours will not make you live forever. However, if eventually the human race understands the ins and outs of telomere biology, we just might.


Recent Comments