Take a moment to look straight ahead. Perhaps you see the face of a loved one. Their face is clear and bright, and you can make out fine details, perhaps a quizzical smile back as they wonder: “why the stare down?” For people experiencing macular degeneration the view is quite different. Instead of a sharp image in the center of your field of view, only a blur remains.
Life would be quite different, simple tasks available to us now would be made impossible. Perhaps most frightening is that this disease, called macular degeneration, can progress to the point of blindness in advanced cases. It generally occurs in aging people where the macula, a spot on the retina where light gets focused for central vision, gets damaged. The retinal pigment epithelial cells deep in the retina stop functioning, and the rod and cone cells die. In recent years, advances have been made in stem cell research that provide hope of a cure for those with this disease.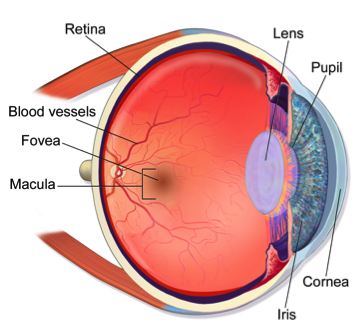
Stem Cells?
Few topics in medicine are more controversial and elicit a more visceral response than stem cells. However, stem cells intrinsically have no unpleasant connotation. In fact, we all have many of them throughout our bodies right now! Stem cells are the mechanism by which our bodies produce and regenerate everything from our skin, to our retinas. What makes them special is that they are undifferentiated, and depending on location, can transform into a variety of cells with specific tasks. Unfortunately there is a limit to the variety of cells a stem cell found in you or me can differentiate to. The stem cells we have in our bodies, called somatic stem cells, are already partially differentiated, and must remain a part of their original tissue. Skin stem cells to skin cells; eye stem cells to eye cells. Even with this limitation, the variety of possibilities for stem cell growth and differentiation for the regeneration of tissue is extraordinarily exciting.
Treatment Options
Stem cells in the eye can be readily found in the front of the uveal region of the eye. The uvea contains the iris, ciliary body, and choroid.
Harvesting some of these cells can be done by a surgeon, after which they can be cultured in a lab to grow and multiply. Culturing stem cells has proved tricky in the past. In traditional cell cultures, stem cells commonly die, or differentiate in culture, losing their key capability, to differentiate to a variety of cells in tissue. One relatively new method is to grow them on hydrogel. The hydrogel mimics basal membranes stem cells are normally attached to, and maintains their potency to differentiate. Once enough cells have been developed, a surgeon can inject a solution of these cells behind the iris at the macula. Once in the retina, the cells should be stimulated to take up the roles of retinal stem cells by inductive interaction with neighbouring retinal cells. If the newly injected stem cells accept their new environment, they can resume the task of the previous retinal pigment endothelial cells and begin producing new rod and cone cells in the once damaged macula. In a relatively short time, vision can be fully restored to those who had lost it. With the perfection of this method, the leading cause of blindness in people aged 60 and over could be made a remnant of the past.
Recent Comments