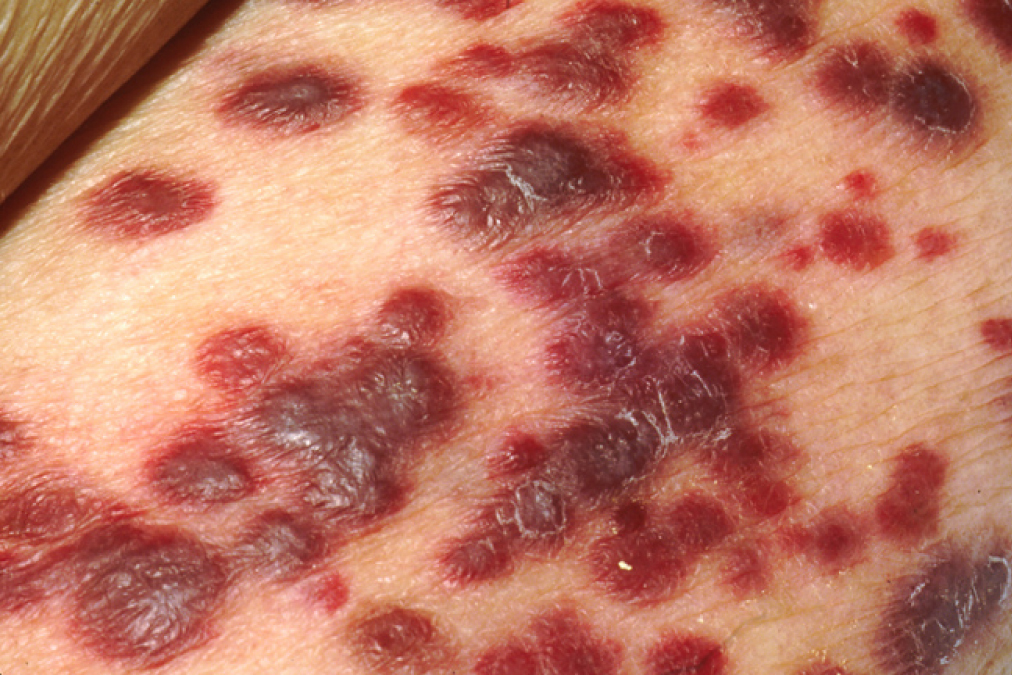
Kaposi’s Sarcoma Lesions By OpenStax College – Anatomy & Physiology, Connexions Web site. http://cnx.org/content/col11496/1.6/, Jun 19, 2013., CC BY 3.0, https://commons.wikimedia.org/w/index.php?curid=30148354
As a human, we believe we are the most superior, intelligent, and evolved species across the globe. How then, are humans still plagued by disease? This can be answered by the fact that disease-causing organisms are constantly evolving, outwitting our own response mechanisms that combat their presence. Our immune system battles these disease-causing organisms by killing our own cells! This programmed cell death is called apoptosis, which not only occurs when we are attempting to rid ourselves of a parasite, but also during general maintenance of our body.
The family of herpesviruses has however, found away around this immune mechanism. The herpesvirus can efficiently infect cells or remain latent within the host cell for long periods of time, enabling the virus to cause lifelong infections (Prasad et al. 2013). Lytic replication can be activated during latency, even after many years. Initiation of lytic replication can be triggered by activation signals such as proinflammatory cytokines, activators of signal transduction cascades, and by agents that alter chromatin structure (Prasad et al. 2013). Apoptosis of the host cell is a threat to the latent herpesvirus because the virus may not complete production of viral progeny before the apoptotic process is completed, thus the inability to infect new cells arises (Prasad et al. 2013). The Kaposi’s sarcoma-associated herpesvirus (KSHV) has evolved to include mechanisms of apoptosis prevention into its genome.
KSHV includes in its genome viral homologs of Flice-inhibitory protein, antiapoptotic homologs of Bcl-2, and a glycoprotein homologous to survivin (Prasad et al. 2013). KSHV latent genes can proficiently modify or hijack host cell molecules to evade our defense system, including those for transcriptional blocks, DNA damage responses, interferons, apoptosis, autophagy, natural killer cells, and adaptive immune responses (Paudel et al. 2012). These molecules also act to inhibit the KSHV lytic cycle and ensure uninterrupted viral reproduction (Paudel et al. 2012). A considerable amount of genomic resources are dedicated to prevent the host cell from undergoing apoptosis, however these efforts are still not perfect; an alternative replication pathway offers the virus a chance of reproducing before the completion of apoptosis. KSHV can detect that the host cell is undergoing apoptosis and adopt an emergency escape alternative replication program (ARP) (Prasad et al. 2013). This pathway is characterized by dependence on caspase-3, the absence of requirement for the replication and transcription activator protein, the product of open reading frame 50, an accelerated pattern of late gene expression, and the production of large amounts of virus, although with decreased infectivity (Prasad et al. 2013). Caspase-3 is an essential factor in sensing apoptosis for KSHV and in the initiation of ARP (Prasad et al. 2013).
It always comes back to cancer, doesn’t it? Several viruses, including KSHV, are recognized as casual agents of specific types of cancer. KSHV, as you can tell from the name, is a herpesvirus associated with Kaposi’s sarcoma, in which it is the primary etiological agent (Suffert et al. 2011). Kaposi’s sarcoma is a highly angioproliferative tumour that is greatly vascularized, probably arising from endothelium and developing primarily in immunocompromised individuals (Suffert et al. 2011). The tumour has a microenvironment abundant with inflammatory cytokines, angiogenic molecules, and growth factors (Paudel et al. 2012). KSHV infection is also associated with aggressive lymphomas and multicentric Castleman’s disease (Suffert et al. 2011).
Viruses encode microRNAs (miRNAs) to regulate gene expression, allowing them to modulate the cellular environment (Suffert et al. 2011). This creates an environment beneficial for its latent intracellular parasitism (Paudel et al. 2012). KSHV encodes 12 miRNAs, clustered around the major latency transcript, K12, which contribute to the inhibition of apoptosis and target caspase-3 as well as Bcl2-associated factor BCLAF1 (Suffert et al. 2011). A major protein during latency, KSHV latency-associated nuclear antigen (LANA-1), is involved in tethering the viral genome to the host chromosome, and binding to and downregulating the functions of crucial host tumour suppressors including p53 and Rb, as well as KSHV lytic switch protein RTA (Paudel et al. 2012). This protein thus promotes viral and tumour growth within the host, as its defense mechanisms to rid the body of viruses and uncontrollable cells are repressed.

Intraoral Kaposi’s Sarcoma Lesion By Photo Credit: Sol Silverman, Jr., D.D.S.Content Providers: CDC/ Sol Silverman, Jr., D.D.S., University of California, San Francisco – This media comes from the Centers for Disease Control and Prevention’s Public Health Image Library (PHIL), with identification number #6058. Public Domain, https://commons.wikimedia.org/w/index.php?curid=827237
KSHV, and many other herpesviruses, have adapted and evolved numerous ways to evade our immune system, excelling at causing disease. These findings in which KSHV undergoes an alternative replication pathway initiated by apoptosis of the host cell, where a high volume of virus with decreased infectivity are produced, provides insight into implications of disease and treatments that stimulate apoptosis, such as chemotherapy. Commonly used chemotherapy treatments activate the latent virus by inducing apoptosis of the host cell, promoting the virus to undergo this alternative replication process. This has beneficial clinical implications, as patients undergoing treatments that induce apoptosis can also be treated with antiviral drugs to reduce any possible infections.
Recent Comments