Aging seems to be defined in many different ways. Some say that age and disease are related as aging is the largest risk factor of chronic diseases. In a biological perspective, aging is describe as senescence or a functional decline in a whole organism throughout time which may lead to an increase to susceptibility to disease and eventually death (1). Senescent cells, which are cells that cease to divide, were known to be the biological cause of aging. Dysfunctional senescent cells does so by disrupting normal tissues as they accumulate in large numbers (2). But commonly, others would say that aging is attributed with the sagging of the skin, wrinkles around the corners of the eyes or appearance of gray hairs.
Because aging is commonly depicted as changes in skin appearance, structure or texture, cosmetic companies race to continually develop the best anti-aging skin products such as ointments, creams, lotions, soaps supplements or procedures to maintain a healthy smooth younger looking skin.
The Metabolic Enzyme potentially involved in the Skin’s Aging Process
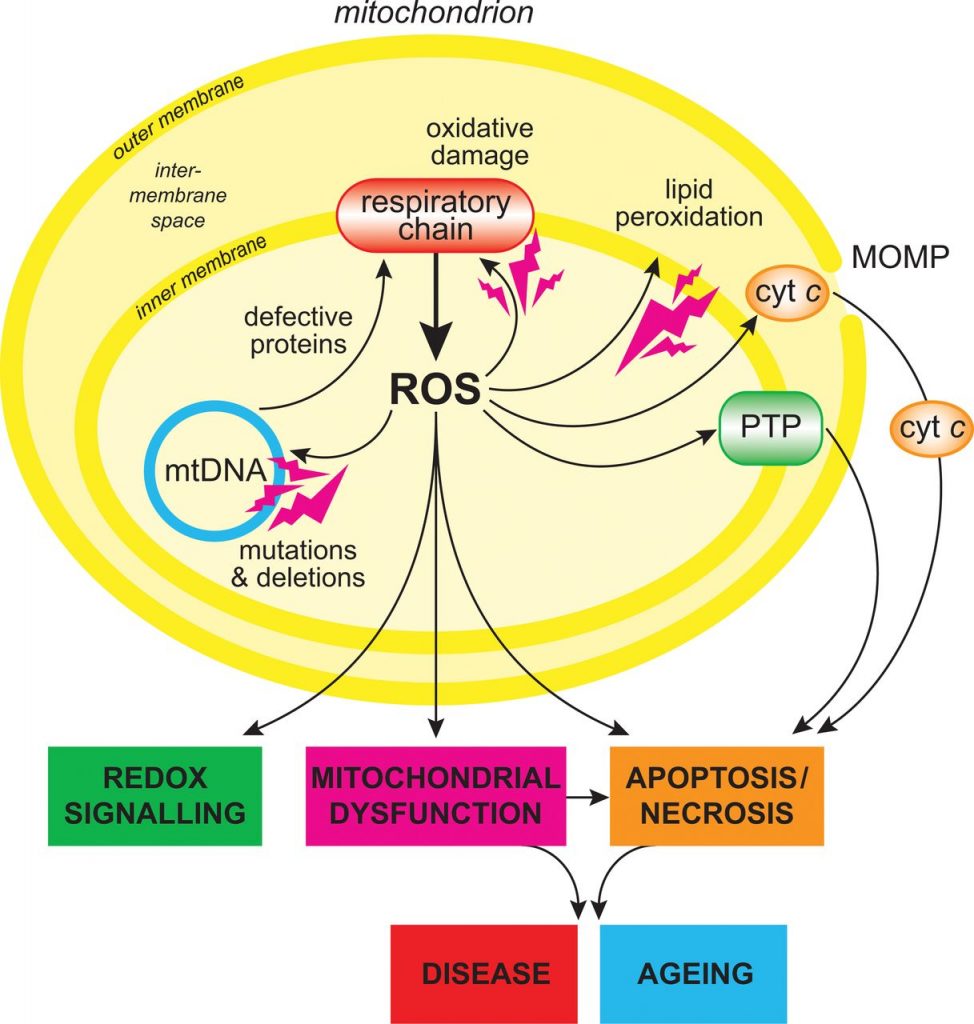
More than 40 years ago, it was hypothesized that mitochondria was the key player in the aging process. It was primarily because most of the endogenous reactive species (ROS), which damage the cells by oxidative stress, are produced by these organelles as a byproduct during respiration. ROS may damage DNA or lead to a dysfunctional mitochondria.It was also because high level of mitochondrial dysfunction, DNA damage and ROS generation are observed with the increase of age. Yet, the exact role of mitochondria in the process of aging remains to be unknown (1).
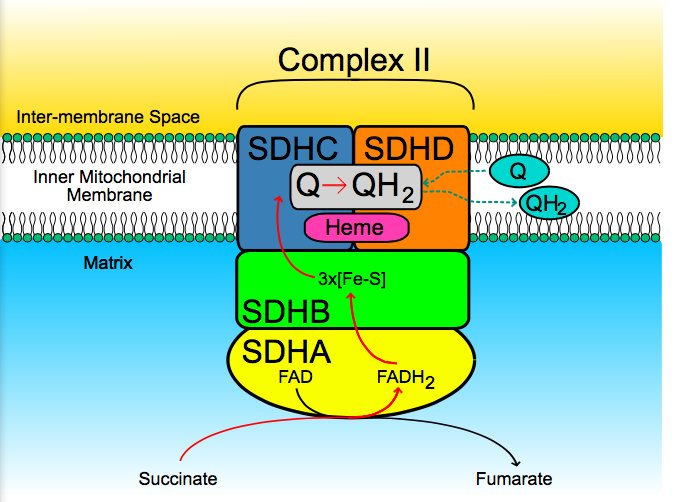
Fig 1. The Mitochondrial Complex II
Source: https://upload.wikimedia.org/wikipedia/commons/5/5a/SuccDeh.svg
Until recently, various researches implicated a possible role of mitochondrial complex II (MCII) in the aging process, specifically in the skin’s aging process. MCII is the smallest out of the four mitochondrial complexes and is composed of four succinate dehydrogenase complex subunits (SDHA, SDHB, SDHC, and SDHD). Recent works has shown that MCII can produce ROS to a similar extent as mitochondrial complex I and III in a rat skeletal muscle and may play a role in generating ROS in human skin cells (1). Other works implicated that mutations in the SDHC and SDHB subunit of complex II accelerated aging and decreased the lifespan of the model animal Caenorhabditis elegans. In addition, complex II has been linked to the process of senescence. In a study of mice with knocked out mitochondrial superoxide dismutase, complex II activity decreased and aging was accelerated(1).
Figure 2: Mitochondria showing ROS production and pathway
Source: Michael P. Murphy. http://www.biochemj.org/content/417/1/1
In the most recently published study on MCII, Bowman and Birch-Machin (2016) used human skin as a model tissue to study the relationship between MCII and human skin aging. According to this research, as we age the enzymatic activity of our MCII in the mitochondria of our skin cells decreases. The researchers measured MCII activity in skin fibroblasts derived from 27 donors with ages 6-72 years old. MCII activity was measured and compared with other complexes. The results were promising. The researchers found that complex II activity decreased in human skin fibroblasts with age. When complex II and complex IV was compared, only complex II activity decreased with age. This result suggests that the decline in MCII activity is specific to human skin cells. However, it was interestingly found that complex II activity decreased only in senescent cells. The evidence showed that skin fibroblast senescent cells in older individuals have less efficient MCII activity compared to senescent cells in younger individuals, which could have implications in understanding the potential causes of the decreased in cellular efficiency as an individual age. In addition, the levels of ROS were observed to be higher in senescent cells compared to the nonsenescent cells. The authors postulated that because ROS levels are elevated in senescent cells, there is a large possibility of increase in mitochondrial and nuclear DNA damage, mitochondrial dysfunction and a possible decrease in MCII activity. It could also be possible that skin senescent cell damage in older individuals is elevated because as we age, antioxidants levels are lowered and age –related senescent cell removal system such as immune system and lysosomal pathway activity declines(1).
One step closer in counteracting the signs of skin aging
Skin is the largest organ of the body. It acts a barrier to infection toxicity and ultraviolet radiation. Understanding the aging process of this organ not only allows for discovery of compounds to maintain dermatological health but also allows skin as a model organ in studying aging in other body tissues (3). With these recent findings, we now have a specific biomarker in developing the next big things in the cosmetics industry, namely anti-ageing creams or treatments. This finding would also pave way for development of drugs to treat age-related conditions such as Alzheimer’s or even cancer.
As for my thoughts, this could just be a beginning in understanding the factors or organs involved in human skin aging. Future research papers may arise to refute or support the idea of MCII complex as a key player in skin aging. Yet, one thing is for sure, the cosmetic industries will continue to develop new trends or products in inhibiting the signs of skin aging or in delaying the aging of our skin.
References:
1.Amy Bowman, Mark A. Birch-Machin. Age-Dependent Decrease of Mitochondrial Complex II Activity in Human Skin Fibroblasts. Journal of Investigative Dermatology, 2016; DOI: 10.1016/j.jid.2016.01.017
2.Campisi,J.Senescent Cells, Tumor Suppression, Tumor Supression and Organismal Aging: Good Citizens ,Bad Neighbors.Cell Press, 2005; DOI: http://dx.doi.org/10.1016/j.cell.2005.02.00
3.Newcastle University. “Scientists make significant anti-aging breakthrough.” ScienceDaily. ScienceDaily, 26 February 2016. <www.sciencedaily.com/releases/2016/02/160226080947.htm>.
Recent Comments