Everyone loses approximately 30 to 40,000 cells per minute. I’m currently losing dead skin cells as I type away on this keyboard. No one really thinks about that fact and no one cares about how many cells they lose per day because it’s not like we’re going to run out of them and be left with a pile of bones. Forget metaphors, imagine being a literal skeleton in the closet. Hypothetically, let’s say that someone approached you and asked you if they could swab your items for some skin cells for their collection (not sure who would go up to people asking them for their dead skin cells, but just go with it) –you wouldn’t care right? I mean, why would you care? It’s not like you go about your day trying to preserve your cells that slough off without remorse and then hold a funeral for them at the end of the day.
So now that we can all agree that we don’t care about who takes our dead skin cells, what if those skin cells possessed such a wondrous characteristic that made them unique from every other cell in the universe? And what if these cells were taken from you without your consent to advance the world of medical research in such a way that would be unimaginable otherwise? That is precisely what happened to Henrietta Lacks.
A brief history
Henrietta Lacks was diagnosed with cervical cancer in 1951 at the Johns Hopkins Hospital in Baltimore, Maryland, when she was 30 years old. She passed away 8 months later, that same year. For cancer research purposes, tissue samples taken during her cervical biopsy were given to the pathology department and the Tissue Culture Laboratory at the hospital. Dr. George Gey, the director of the laboratory, discovered that a particular set of cells were growing more robustly and were more durable than had ever been seen before. These cells were named HeLa after Henrietta Lacks. Data with HeLa cells was published in 1952, and with that came many requests for the first ever immortal human cell line. Soon, HeLa cells were cloned and supplied to researchers all over the world.
…and of course some controversy
Although the cells were termed HeLa after Henrietta Lacks, the public, including her family, lacked this knowledge (pun intended). It wasn’t until 1973 that a scientist contacted her family in search for more tissue and blood samples. Legal and ethical implications arose because the public came to know that Henrietta’s cells had been cloned and sold all over the world (~$250/vial) without her or her family’s consent. Meanwhile, her family had been living in poverty and without healthcare. The story sparked so much media attention that it was actually made into a movie: The Immortal Life of Henrietta Lacks.
See how much conflict can be avoided if one simply just asks for permission? It’s really not that hard to do.
Cancer cells and immortality
Cancer cells are different from normal cells in that they don’t experience programmed cell death (PCD), apoptosis or cellular suicide. Cell death is the reason we don’t look like ducks and have webbed toes and fingers (well, among other reasons too of course). Backtracking to the introduction, it’s also the reason we lose thousands of skin cells per minute. Most cells undergo PCD after ~50 divisions. HeLa cells are especially unique in that they’re extremely durable: easy to grow, store and ship. Under certain in vitro conditions, they can divide indefinitely (Figure 1). These characteristics of HeLa cells are also why they’re problematic: they can easily invade and contaminate other cultured lines.
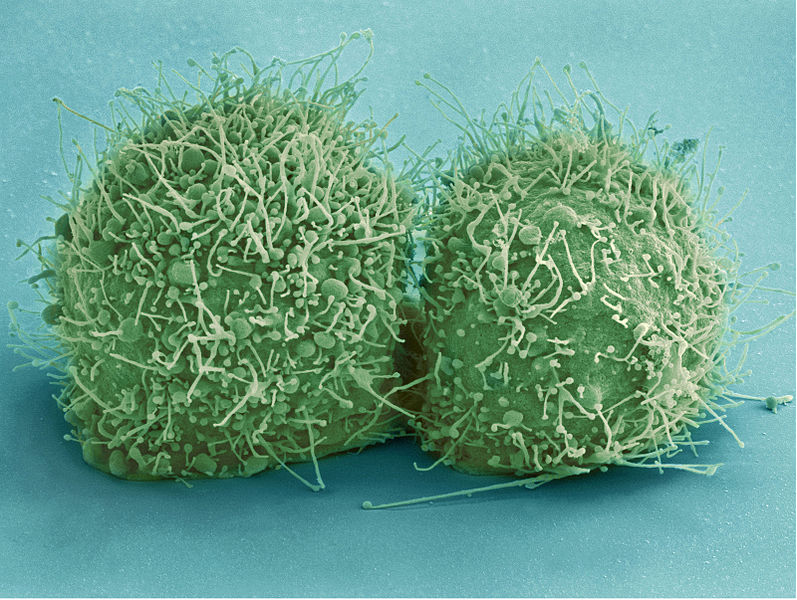
Figure 1. HeLa cells that have just divided: viewed with a scanning electron micrograph. Image credits: Wikimedia Commons, Public Domain
The secret to immortality is telomeres. Telomeres play a role in protecting our chromosomes and they exist on the ends of our DNA strands, much like how helmets are to be worn on our heads so that we don’t break our skulls if we happen to fall off our bikes. Telomeres shorten every time a cell replicates itself and eventually the cell stops dividing because the telomere becomes too short to function adequately (Figure 2). Telomeres are synthesized by telomerase and this enzyme has very low activity in most cells or is switched off in early development. The regulation of the telomerase gene commits cells to senescence. HeLa cells, like other cancer cells, are considered immortal because their telomerase is active during cell division and allows them to surpass the typical cell division limit. Therefore, if scientists can find a risk-free way to stop telomerase activity in cancer cells, that may be the surefire way to cure cancer once and for all. It’s also possible that cancer could be detected by measuring telomerase.
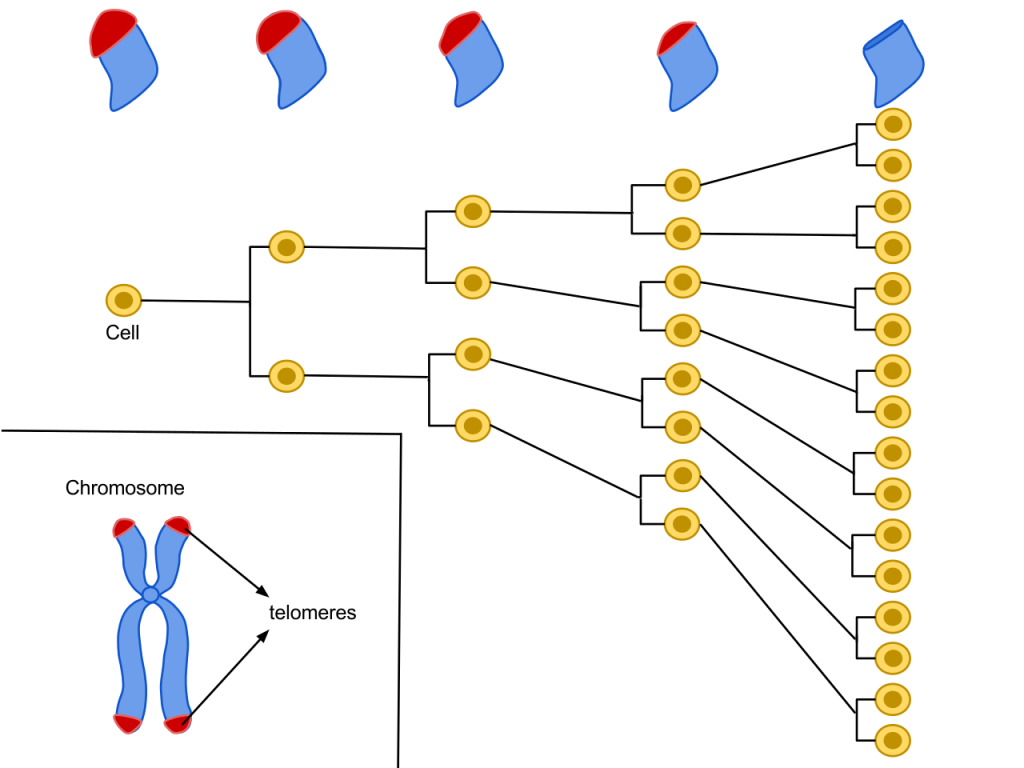
Figure 2. The mechanism of telomere shortening per cell division cycle. Image credits: Wikimedia Commons CC-4.0.
A poor diet, minimal exercise, stress and smoking can also shorten telomeres. Imagine if we could just put on a special hat and a pair of shoes that functioned exactly like telomeres to make us immortal…
Why HeLa cells are HeLLa awesome
HeLa cells have been used extensively in medical research for the past 60 years. The field of virology was actually developed from HeLa cells, as they were infected by every virus in the book to learn how the various viruses would impact the cells. A breakthrough in genetic medicine also would not have been possible without HeLa cells, as their chromosomes were clearly visible after treatment with a particular stain. Human genome mapping also began with fusing mouse embryo cells with HeLa cells to create the first hybrid cell.
A rather interesting point about HeLa cells is that they contain 70-90 chromosomes, whereas Henrietta Lacks’ genome originally contained 46 normal chromosomes. Since a species is identified by its karyotype, and the karyotype of HeLa cells has been subject to many changes, there is an ongoing debate about whether or not the HeLa genome can be considered a human genome.
From being a key component to creating the polio vaccine to proving that cancer cells can grow in space, there’s no doubt that HeLa cells are hella awesome.
Also, it’s kind of unfair that HeLa cells get to be immortal and also got to go to space. Hopefully someone at least discovers the fountain of youth for us next.

Recent Comments