Introduction
We begin our lives as little congregations of proliferating cells. We expand and grow, familiar shapes begin to form, segments become identifiable with functional specificity. Somewhere along, we become “life” although where along is arguable. Eventually we are large-headed tube feeders somersaulting and kicking in the maternal hotel where light is dark and all is warm and worry is a foreign characteristic for outsiders. How ironic that we both leave the womb and enter the tomb with a light at the end of a dark tunnel (unless of course you are born as the “cheeky” type and breech the status quo). Nevertheless, our little life-in-the-works has not acquired the complete “fit for the world” tool kit prior to parturition just yet; after all, “the night is dark and full of terrors” and we are all in need of a protector…or protectors…or 10-100 trillion protectors—yes that seems best. A push, a squeeze, maybe even a plunge, clamp or snip, and we’re off on the trajectory of life yet, without even knowing it, the humblest of protectors have already begun their new life’s work: managing ours.
Figure 1. A cartoon drawing depicting a feisty bacteria ready for a fight. The image is released free of copyrights under CCO Creative Commons. Attribution not required.
It may not be overtly apparent, but we all have microbes (bacteria, fungi, protozoans, archaea) living and dying on us at all times. Humans, among other earthly inhabitants, are ecosystems for microbial communities which gives the terms “microbiome” (the taxa association) and “microbiota” (the list of microbes and their genes) interesting implications—here we are living on, and thus effecting, the Earth and the communities that we share it with and all the while there are symbionts treating us in similar ways. Our unique and individual microbiomes influence, or are influenced by, a mind-boggling amount of processes such as inflammation, anxiety, circadian rhythms and obesity, cancer, alleviation of type 2 diabetes, craving for junk food… the list goes on.
Figure x. A 1756 drawing of Antonie van Leeuwenhoek’s microscope he used in his research by Henry Baker. This image is released free of copyrights under the public domain of the United States. Attribution not required
It may come as no surprise that Robert Hooke and Antonie van Leeuwenhoek, pioneers of the early microscope, are attributed with the first description and discovery of a microorganism in 1665, the microfungus, Mucor. Sounding likely unintentionally adorable, Leeuwenhoek termed the first microbes “animalcules”. Now, with 353 years of advancement and metagenomic sequencing, our understanding is considerably more advanced. There’s an infinite set of questions here, but lets focus on where we actually acquire our micro-residents and some of the effects of acquisition circumstance.
The microbiome one obtains near or during birth may change over time with aging, stress, diet, environmental factors, etc. Focusing on where and when the microbiome develops, we will consider pre-birth (in utero), during birth (vaginal or cesarean), first foods, and environments. The idea that the microbiome is acquired during and after birth has been widely accepted for years which stems in reasoning from the “sterile womb paradigm” that has been considered dogma for over a century. Recently there have been several publications suggesting the existence of commensurable placental microbiomes that a fetus may obtain in utero. This was curious, but more so when discovering a critical assessment and study with more rigorous methodology essentially squashing the placental studies and attributing their results to i) their PCR and next-generation sequencing methodologies not having adequate detection limits for low density populations of bacteria, ii) contamination susceptibility of the methodologies and iii) environment (hospital), and finally, iv) wrongly interpreted stool samples findings—a fine example of the scientific method at play.
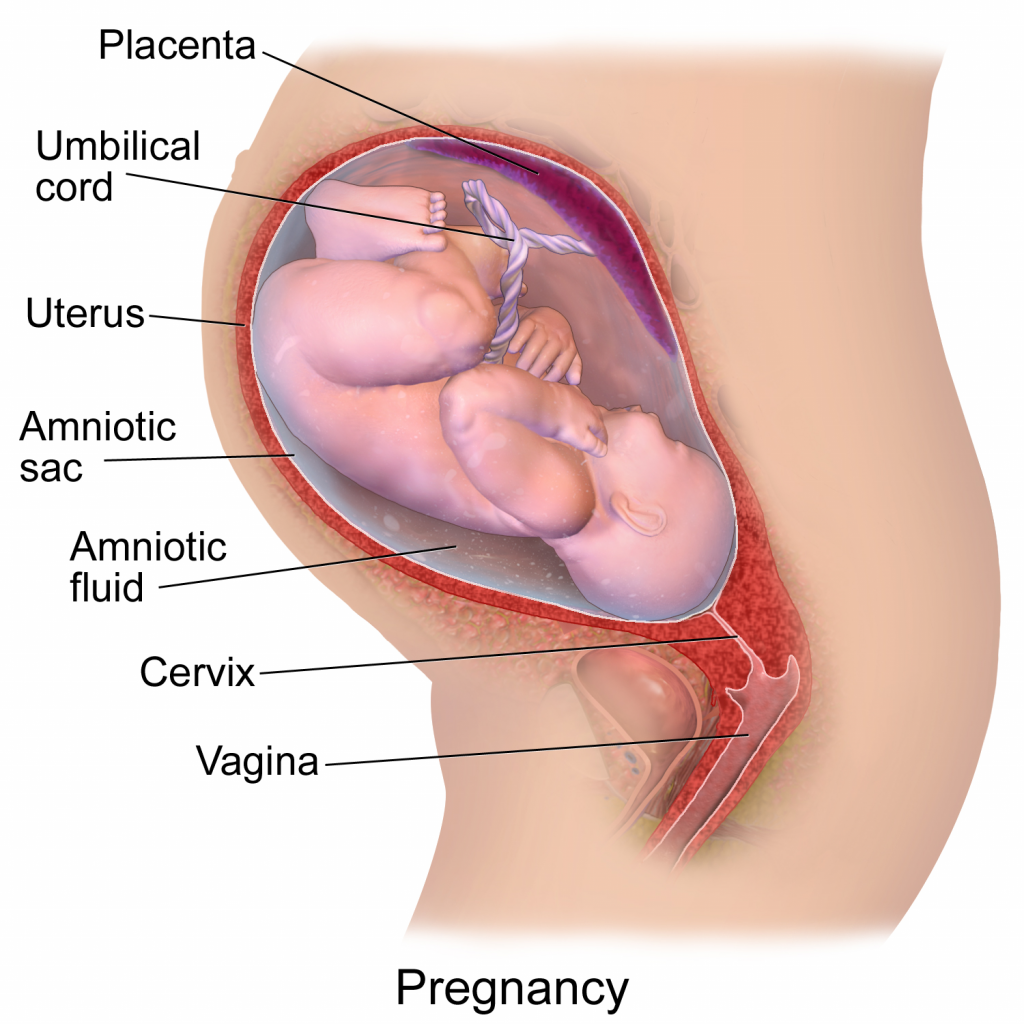
Figure 3. A diagram depicting a pregnancy well into gestational development. This image has been released free of copyright by Blausen.com staff (2014). Own work, CC BY 3.0
Without any intention to discourage necessary C-sections, there are considerable benefits to a vaginal birth, namely attributed to a vaginal microbiota coat applied during delivery. During pregnancy, a woman’s vaginal microbiome will undergo compositional changes by lowering diversity with dominance from spices of lactobacilli, clostridiales, bacteroidales, and actinomycetales. After vaginal delivery, a newborn’s microbiome will strongly reflect the microbiome of the mother’s vagina and feces whereas after cesarean delivery, it is more reflective of the mother’s skin and hospital environment. As we are all aware, hospital environments can be concerning to downright pathogenic at times which is why newborns can acquire pathogenic bacteria like Clostridium difficile, methicillin-resistant Staphylococcus aureus (MRSA), and pathogenic E. coli while lacking beneficial Bacteroidetes and Bi. Longum subsp. Infantis. Beneficial Bifidobacterium occasionally make their way onto newborns during a cesarean birth and when they do, pathogenic E. coli and C. difficile are not found; I think this is awesome, the microbial army your mother can give you will outcompete and destroy pathogenic marauders! For reasons relevant to the microbiome composition, a cesarean birth may increase risk of disease such as celiac disease, asthma, type 1 diabetes, and obesity. It sounds a bit inappropriate and awkward but, would rubbing a cesarean newborn on the mother’s vagina immediately after delivery be a terrible idea? —there’s a story for prom night!
As a newborn, your initial dining experiences play a considerable role in introducing new microbial communities. Breast feeding over formula has many benefits to an infant including the stimulation of neonatal microbiome maturation of the gut. Environments, such as hospital delivery rooms, can have lasting effects also; for instance, you may feel safe laying across your father’s perhaps burly and hairy chest as an infant, and you should because he just gave you another powerful microbial community of his own. Remember that maternal army of microbes that fends off bacterial marauders? The take away is that some species will stay for good, giving you a microbial signature that is unique and, in some cases, bacterial assemblages can be traced back to individual persons. So, what would happen if Neil DeGrasse Tyson, the astrophysicist, and Robert Krulwich from the Radiolab podcast shook hands; would their microbiomes mix, die off, or would they compete and declare a winner? I’ll leave you to find out.
Conclusion:
Whether you get your microbiome from your mother, your father, the foods you eat, the places you go, the people you meet or all the above, you have a microbiome. It influences your mood, your metabolism, your health, and is very much a part of you. Our world is infinitely complex and full of surprises, but I for one am grateful to know that a whole history of biological evolution is hanging out and navigating life with me.

Recent Comments