Sometimes it can be hard to admit that we’re wrong. One of the errors that researchers and doctors make is assuming the cause of disease must originate in the affected organ. At first glance, this assertion seems to make sense. However, the development of disease is insurmountably complex, and more often than not, these intuitive assertions are proven false by the researchers brave enough to challenge well established paradigms. Our current understanding of how disease manifests has changed drastically over time, and researchers are now looking for alternative explanations as to how some seemingly incurable diseases develop and progress.
A current example of how emerging evidence presents a novel cause of disease is in the case of Parkinson’s disease (PD). PD is a neurodegenerative motor disorder characterized by symptoms including muscle rigidity, slowness of movement, postural instability, and tremor. Most patients, however, experience symptoms beyond motor dysfunction including gastrointestinal (GI) symptoms. Interestingly, many of these seemingly “unrelated symptoms” precede symptoms of motor dysfunction by several years. But are these symptoms really “unrelated”?
Advancements in PD research exploded in recent decades as new evidence has materialized. The pathophysiology of PD seems to be well characterized. We know that the motor dysfunction experienced by patients with PD is a result of the accumulation of an abnormal protein called α-synuclein in the dopamine producing neurons of the substantia nigra. Neuronal function becomes corrupted by the accumulation of insoluble clumps of this protein, and symptoms of motor dysfunction inevitably ensue.
The substantia nigra is a cluster of neurons in the midbrain that is functionally part of a brain region called the basal ganglia. These neurons project to a structure called the striatum, forming what is known as the nigrostriatal pathway. This pathway is thought to be cardinal in producing and coordinating movement. Therefore, damage to a component of this pathway will ultimately produce motor dysfunction. However, recent evidence suggests that it is not motor dysfunction that appears to be the first symptom of PD, but rather a spread of non-specific symptoms related to GI dysfunction.
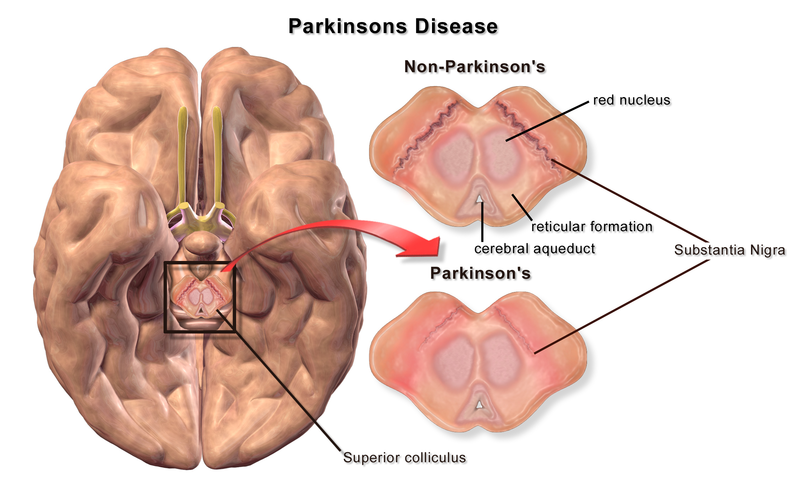
Figure 1. Substantia Nigra in Parkinson’s Disease. Credit: Blausen.com staff (2014). “Medical gallery of Blausen Medical 2014”. WikiJournal of Medicine 1 (2). DOI:10.15347/wjm/2014.010. ISSN2002-4436. This file is licensed under the Creative Commons Attribution 3.0 Unported. No changes were made to the figure.
GI dysfunction is a commonly reported symptom that precipitates the onset of motor dysfunction in individuals with PD. In consequence, the possibility that PD originates in the enteric nervous system (ENS) has recently gained a lot of traction. The ENS is composed of a highly complex neural network in the GI tract that coordinates functions including gastric motility and communication with the brain. Most notably, the ENS and brain are connected through a complex neural network called the gut-brain axis.
The factors that initiate the pathophysiological cascade causing the destruction of SN neurons are unknown. However, a likely candidate may be our nervous system’s interaction with the outside world. Alpha-synuclein is normally expressed in the presynaptic terminals of neurons, and is though to be involved in regulating neurotransmission. Under pathological conditions, Lewy bodies composed of α-synuclein fibrils begin to accumulate. It is thought that α-synuclein could act as a prion like protein, capable of acquiring an “infectious” phenotype that spreads throughout the nervous system, transforming physiological α-synuclein into its pathological from.
Figure 2. Neuron. CC0 Creative Commons. Free for commercial use. No attribution required
In the first stages of PD, these aggregates are initially concentrated in the neurons of the ENS and olfactory bulb. A compelling explanation for the GI symptoms that present prior to motor dysfunction is that aggregates of α-synuclein may initially develop in the ENS and progressively spreads to higher brain regions through the vagus nerve. What triggers the abnormal α-synuclein aggregation has not yet been discovered. However, evidence indicates that pathogens or environmental toxins may trigger oxidative stress and mucosal inflammation, initiating subsequent protein accumulation. Some studies have also shown that this pathological process may be initiated by our own gut microbiota.
We know that individuals with PD tend to have different gut bacteria than healthy individuals. In a study conducted on a PD susceptible mouse model, the mice raised in germ-free environments showed fewer motor symptoms than the mice raised in non-sterile environments. Additionally, when the mice raised in a non-sterile environment were treated with antibiotics, the severity of their symptoms decrease. That is, there seems to be something inherent to their gut microbiome that impacts symptom severity. Finally, the researchers introduced human gut bacteria from PD patients into the GI tact of the same mouse model. These mice began to develop severe motor dysfunction, whereas no effect was seen in mice injected with the gut bacteria of healthy participants.
From the evidence presented in this study, we can be confident that at least in a mouse model, gut bacteria can have a profound effect on PD severity and progression. However, more evidence and replication studies are necessary before we can truly confirm the role of gut bacteria in PD. In the event that this is proven to be true, the way we treat Parkinson’s disease may change completely. Also, if we can identify the strains of bacteria that seem to cause the disease, we may be able to screen patients earlier before they develop irreversible brain damage.
There is a lot we still don’t understand about Parkinson’s disease. However, with PD being the second most prevalent neurodegenerative disease, the possibility of a cure has the power to transform the lives of those affected forever. Current therapeutic approaches work to curb the motor symptoms, but they do not stop the brain damage or address the non-motor symptoms. Although provocative, the assertion that we have been approaching PD wrong this entire time seems to be more convincing than not. However, it is important to keep in mind that animal studies don’t always translate to humans.
Sometimes, even the simplest of experiments can completely transform our understanding of physiology and disease. These discoveries provide hope for many that a cure may soon be on the horizon for various “incurable” diseases. In the scientific community, however, these pivotal discoveries foster in us a sense of humility that allows us to open our minds to the transformative possibility of being wrong.


Recent Comments