Introduction
You are a young high school or university woman living in Poland in 1941, just arrested for your Jewish ethnicity by the German Reich and transported and confined to the Ravensbrück Concentration Camp, approximately 90 km north of Berlin, Germany. About a year of horror, tragedy, and suffering passes and out of your deplorable condition, you are condemned to become one of 74 Polish female medical test subjects of SS Professor Karl Gebhardt who wishes to study bone, muscle and nerve regeneration to enhance soldiers of the Nazi Regime.

Figure 1. Chalked on X’s mark females as prisoners at the Ravensbrück Concentration Camp to be transported by the Swedish Red Cross at the end of World War II.
This image has been free of copyrights under the Public Domain by the Swedish Red Cross.
Under little to no anesthesia, samples of bone, muscle and nerves are removed and virulent bacteria are introduced leading to unimaginable pain, mutilation, and permanent disability if you happen to emerge as a survivor of the procedure and post procedural executions. Personally, I cannot begin imagine this reality with due diligence. The Nazi Medical experiments of WWII aimed to advance science and medicine in the cruelest and most unethical manner imaginable, especially when compared to today’s standards and, without implying any justification, I also understand how important the study our nervous system can be for those afflicted with any serious neurodegenerative disease. But, how do we administer tests on human nervous tissue from the peripheral nervous system (PNS) and central nervous system (CNS) when taking biopsies almost certainly leads to permanent damage—while keeping such ethical regulations as to prevent history from repeating itself? The answer lies with some clever tweaking of the cellular machinery in blood cells or skin cells to induce cell fate conversion from the selected cell type to lineage-specific nervous tissue that may be studied in a laboratory setting.
I have been rather hooked on the concept of personalized medicine since it has first come to my attention—it just makes sense, people are different and respond differently to treatment. Nervous tissue of the PNS and CNS is extremely fast growing during embryonic development but reaches a stage where such growth must be suppressed; this creates numerous barriers and challenges in forming methodology for repair or prognostics, yet ground breaking understanding and methods are continuously on the rise. The overwhelming implications and successes of stem cell research circulating the literature over the past few decades have given us an entirely new view of regeneration potential, but not without ethical concern. An injury to the spinal cord is swimming in an extracellular matrix of inhibitory myelin proteins and lacks the necessary regenerative neurotrophins (growth factor). Many of these barriers have been successfully overcome in primates with the use neural progenitor cells (NPCs) derived from human embryonic spinal cord stem cells. Because of the ongoing embryonic debate (such as, “at which point does a cluster of cells become a human?”), the ethnicity using of embryonic stem cells remains questionable.
From Blood to Neurons
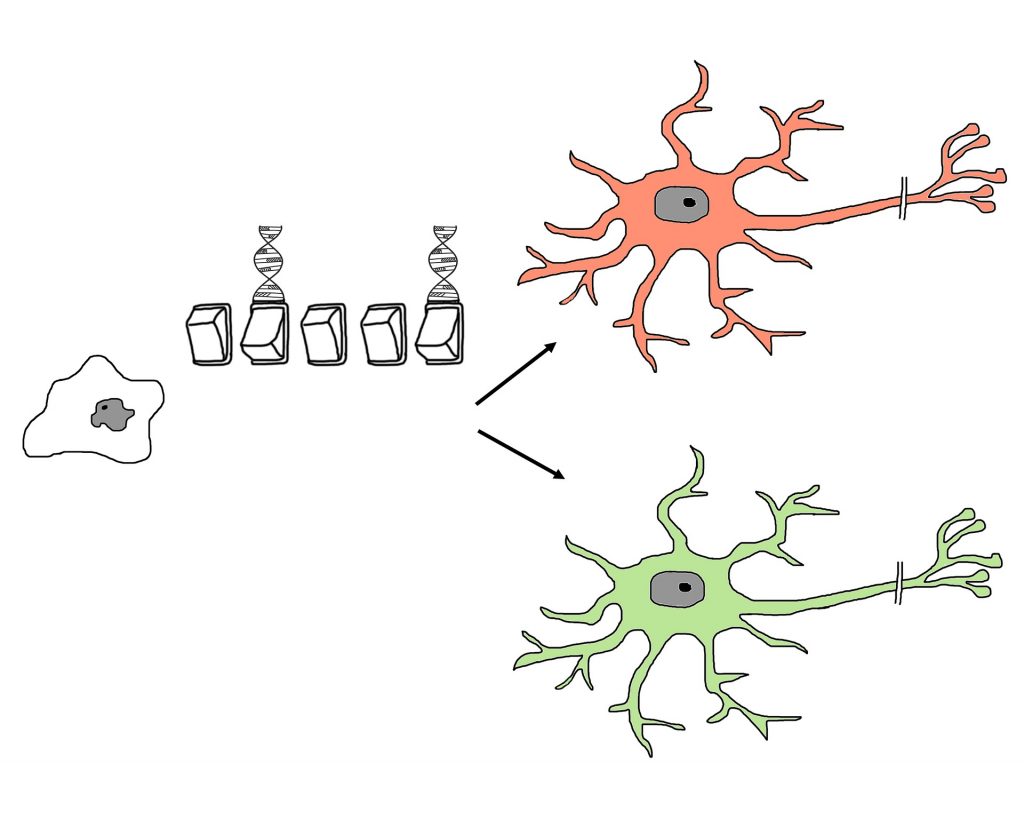
Figure 2. Artist’s conceptual depiction of a stem cell differentiating into two type of neurons, determined by molecular switches controlling gene expression. The image is released free of copyrights under CCO Creative Commons. Attribution not required
Mick Bhatia, director of the McMaster Stem Cell and Cancer Research Institute in Canada, and his team recently published their work in Nature where they induced direct cell fate conversion on adult human peripheral and neonatal blood cells with a single-factor OCT4 reprogramming conversion to tripotent induced neural progenitor cells (iNPCs), intermediately formed as neural stem cells. Because of their work, we can now, in laboratories, grow neural cells of the PNS or CNS in a dish from a simple blood sample that is precisely patient specific. What’s more, this technique has been shown to be successful on cryopreserved blood meaning we could look into mechanisms of ailments of patients from the past whose blood has been stored. Some questions we might answer are: why do some feel pain where others feel numbness; can neuropathy be predicted in Type 2 diabetics; what drugs are going to specifically illicit the desired response for pain management and what should be developed? In the past 5 years, 29% of Canadians have used prescribed non-specific opioids for pain management, 66.5-78.1% in the USA, and as of 2016, 94.3% of countries with available data are shown to prescribe the same. The non-specific opioids prescribed have addictive proprieties, undesirable side effects, and massive economic influence, but with virtually risk-free potential to study individual’s neural mechanics, we might overcome such social epidemics in the near future.
From Skin to Neurons
As mentioned, stem cells are awesome, but certain procedures pose ethical implications. It turns out a team from the Washington University of Medicine in St. Louis recent published in 2017, a way to growing neuronal CNS tissue from human skin cells (fibroblasts) in a dish without ever converting to a stem cell. The methodology involves inducing the fate conversion by implementing a “proper chromatin environment” with miRNAs, which alters the configuration of chromatin for expression and DNA methylation, in conjunction with the default neuronal state being induced with mRNA expression. Sr. author and assistant professor of developmental biology Andrew S. Yoo, PhD, describes this process as being “…more like a renovation… [where as] …going back through a pluripotent stem cell phase is a bit like demolishing a house and building a new one from the ground up.” They were able to model the linage-specific neuronal reprogramming by expressing the connection between miRNAs and neuronal subtype-specific transcription factors successfully.
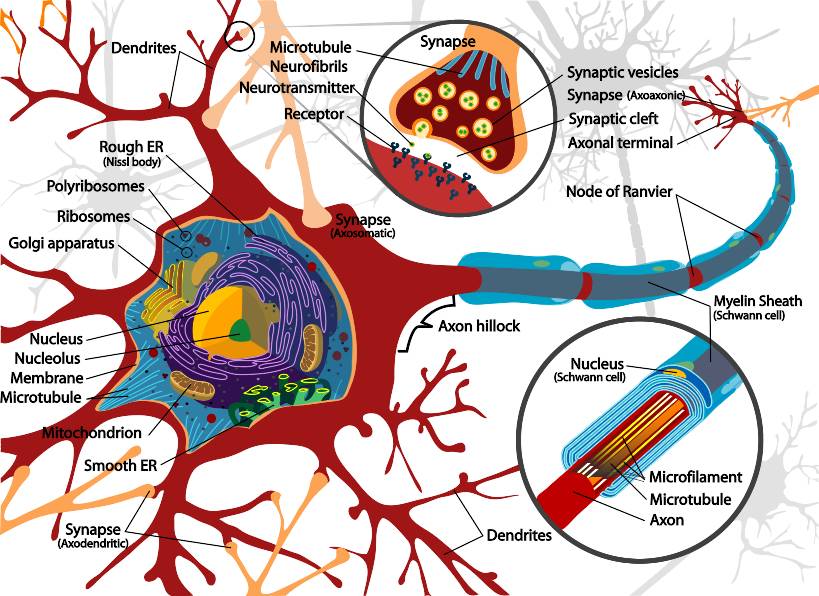
Figure 3. Complete diagram of the fundamental components of a myelinated neuron. This work has been released into the public domain by its author, LadyofHats
It is my hope, and the hope of many, that with these new methods of personalized medicine on systems as complex as the human nervous system, we will gain insight with acute detail into the mechanisms of many aliments and afflictions that affect nervous tissue, and how and why it differs from person to person. Let us tackle diseases like Parkinson’s, multiple sclerosis, amyotrophilic lateral sclerosis, Alzheimer’s, Huntington’s, peripheral neuropathies, and spinal muscular atrophy head on, without guessing and without a pharmacy of management pills in the patient’s bathroom cupboard. Let us be remembered by history as ethical, respectful and successful and those who had learned from our own past to make the world a better place.

Recent Comments