Diabetes Background
Diabetes is a common, debilitating disease defined by a low serum insulin, causing hyperglycemia (high blood sugar). In 2017 alone, it was estimated that over 425 million people around the world are living with diabetes, which caused an estimated 727 billion USD in healthcare costs. There are two main forms of diabetes commonly observed, with similar symptoms but very different etiologies. Type I diabetes is classified as an autoimmune disease, in which the body’s own adaptive immune system targets and kills the insulin producing b-cells of the pancreas. People with type I are unable to produce their own insulin. It is most commonly observed in children and most believe it is mostly has a genetic cause, yet environmental factors are believed to strongly influence the disease’s onset. A strong discordance (one twin has the disease while the other does not) is observed in monozygotic twins, with a concordance rate of only 27.3%. This strongly supports the idea that epigenetic variations through variably histone methylation (gene silencing) can contribute to the diseased state, and that an individual’s environment and experiences have just as strong, if not stronger, role in the disease’s etiology. Type II diabetes is characterized by a similar lack in insulin production as type I, but the cause is much different. Type II results from insulin resistance, which essentially causes the b-cells to work in overdrive, and wear out, becoming damaged and stopping insulin production all together.The main therapy for both diseases is supplemental insulin injections, yet these can be draining and difficult for many to manage. In current diabetes research, there are two main fields of study, regenerative therapies aimed at increasing the abilities and proliferation of existing b-cells, and stem cell therapies aimed at producing insulin-producing b-cells from either human embryonic stem cells (hESC) or induced pluripotent stem cells (iPSC) to replace and supplement existing, damaged b-cells.
by: Sanador2.0 (CC Attribution 4.0 International)
Regenerative Therapies
Regenerative therapies are based around the idea of using what is already there to replace what is being lost. This leads to two main areas of research, 1) increasing the b-cells ability for self-replication, 2) transdifferentiating other, neighbouring cells into b-cells.
- Targeting b-cells themselves to activate proliferation is an enticing target, as would act as a preventative measure against b-cell death. These cells are believed to reach senescence and to even die off within type II diabetes, because the hyperinsulinemia seen in the pre-diabetic state (excess serum insulin levels) requires both hyperplasia and hypertrophy of the b-cells. This allows accumulation and/or higher mutation rates. Specifically, the p53 tumour suppression pathway is upregulated in type II diabetic b-cells, resulting in increased rates of cell arrest and apoptosis. So, essentially if a way to protect the b-cells from this damage can be found, it will allow for continued islet growth and insulin production. It won’t combat the insulin resistance at the sole of the disease, but it will allow the body to be able to produce and adapt to an increased requirement for insulin. The challenge seen in type I is the autoimmune attacks, which even if replication can be upregulated, the resulting cells will still be attacked by the immune response. Various compounds that can induce b-cell proliferation have been identified, however, many have only been observed in animal models or human cell cultures. This leaves the need for more research, to determine the effect that these substances could have on other tissue types, if provided from an exogenous source.
- The transdifferentiation of existing cells into insulin producing b-cells is another target of regenerative research. The pancreas has two functions, as an exocrine organ, producing digestive enzymes, and as an endocrine organ, regulating bodily metabolism through hormone production. Cells from both areas of function have been shown to transdifferentiate if needed. Within the exocrine portion, ductal cells (epithelial cell of the pancreatic duct) and acinar cells (digestive enzyme producing cells) have both been shown to turn into insulin producing cells. The ductal cells, which are less specialized, have been shown to differentiate into insulin+ The acinar cells are a bit trickery however. As these exocrine cells are highly specialized, they essentially “dedifferentiate” into ductal cells, and from there into insulin+ cells. Excitingly in recent years, acinar cells have been shown to be able to transdifferentiate into islet progenitors, with the ability to differentiate into all islet cell types, including b-cells.
Stem Cell Therapies
Stem cell research into diabetes has become a major focus for many labs around the world. The idea they present is the production of functioning, islet b-cells, from undifferentiated stem cells. Just like for regenerative therapies, there are two predominate focuses of research: 1) the use of hESCs, and 2) the use of iPSC.
- hESCs and their use are a morally distraught area. The major challenge to their use is the issue of life, and the fact that they require a fertilized human embryo. Misconceptions are common however, such as that they generally come from abortions. In fact, hESCs are sourced from fertilized blastocysts donated during in vitro fertilization procedures. Regardless of the ethical dilemmas, it cannot be denied that hESCs offer researchers the ability to literally grow new tissues. Various labs have tried to develop b-cells from hESCs, but initial trials only resulted in polyhormonal cells, which produced small quantities of a range of hormones, including insulin. When transplanted into animals, these polyhormonal cells were unable to alleviate the hyperglycemia seen in the animal models. More recently though, several labs have been able to develop insulin producing cells that closely resemble b-cells in their gene profiles. Using these cells, studies have shown prevention of diabetes in pre-disposed mice models, and reversal of the hyperglycemia in induced diabetic mouse models. The problem with using foreign hESC grafts is the risk of rejection, requiring individuals to use constant immunosuppressants. Additionally, in type I diabetics, the induced b-cells could still possibly be subjected to autoimmune attack. Various clinical trials using hESCs are underway, specifically one by the company ViaCyte. Their trial is utilizing hESC derived b-cells grafts, surrounded by a capsule that inhibits autoimmune attack and rejection of the graft. The graft is not being tested in type II diabetics, as many individuals with type II additionally experience other health complications and take medications for those, which ViaCyte is unsure of how they would interact with the graft.
- iPSCs are a form of pluripotent stem cell similar to hESCs, but they are derived from an individual’s own, normal somatic cells. iPSC use appears to offer less of a moral problem compared to hESCs, however, they do still exist. Methods of iPSC production generally use transfection of a somatic cell with a virus, that has been engineered to “reintroduce” various protein expressions required for pluripotency. These methods can produce varied results however, with the new expressions and/or incomplete demethylation resulting in failed differentiation and even autoimmune rejection of the cells. Additionally, if the cells are being used due to a genetic disease cause, additional gene editing may be required to “fix” the deleterious gene. iPSC derived b-cells would offer a possible transplant to avoid rejection in type II diabetics if they can be produced with negligible damage from the transfection method. Cases have been reported where iPSC derived b-cells have been developed successfully, which would offer a therapy for type II diabetics.
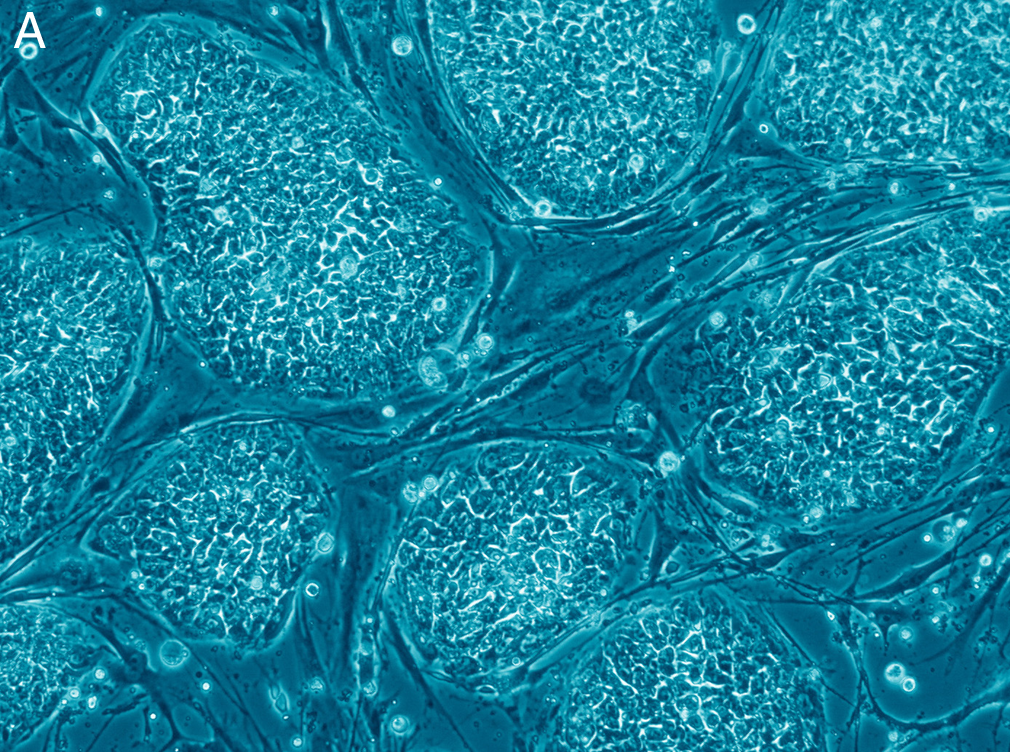
by: Vojtech Dostal (CC Atribution 2.5 generic)
Conclusion
As diabetes is generally diagnosed after irreversible damage has already been done to the islets, constant insulin treatment becomes a necessity. However, as research into the fields discussed continues, scientists are getting closer to the developments of treatments that will alleviate the need for insulin injections, dramatically improving quality of life.

Recent Comments