In the last few decades, the human race has made incredible strides in the realm of organ transplantation. Organ transplantation, once a primitive science experiment, has developed into the most effective treatment available for end-stage organ failure. We have progressed from grafting thin and simple tissue types like skin, to complex organs like hearts, livers and even faces! Moreover, graft and patient survival has improved tremendously due to improved patient selection criteria, surgical techniques, organ preservation, infection prophylaxis, and immunosuppressive drugs. However, despite these lifesaving advances, there are still plenty of complications that can lead to devastating outcomes.
The Bad News…
The harsh reality of organ transplantation is that it is a victim of its own success. Despite it’s effectiveness, it increases morbidity and mortality of patients worldwide. As the population ages, the prevalence of chronic disease has increased and therefore, so has the need for organs. As of 2014, 4500 people in Canada were awaiting transplants, and 278 of those individuals died in the process. This may not seem like bad odds. However, receiving the organ and undergoing major surgery is only half the battle.
One of the most significant complications associated with organ transplantation is graft rejection. Ironically, this is caused by the system that wants to protect us the most, the immune system. The primary function of the immune system is to protect the body from internal and external threats like bacteria, viruses and cancer cells. Due to the fact that the immune system needs to find and destroy a broad range of invaders, it is essential that the immune system can discriminate between self and non-self to avoid attacks on the body’s own tissues. One of the main contributors to graft rejection in mammals is a series of genes that code for Major Histocompatibility complex (MHC) antigens. With the exception of identical twins, all individuals have different DNA and therefore, express distinct MHC peptides on their cells. Therefore, the organ donor and recipient do not have the same MHC antigens. As a result, the immune system of the recipient recognizes the donor tissue as a foreign threat that must be destroyed.
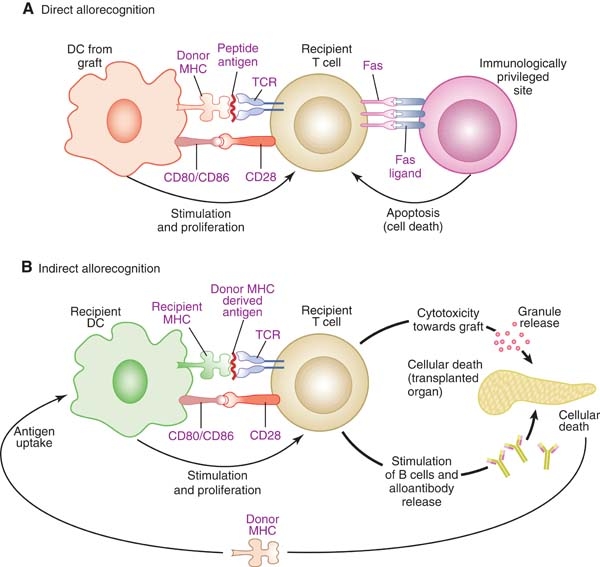
Direct & Indirect pathways of graft rejection. By: Drukker, M., Permission: CC BY 3.0. Not Modified
This can occur via two major pathways: the direct and indirect pathways. The direct pathway results in acute allograft rejection. This process occurs when recipient T-cells interact with the MHC antigens on donor immune cells causing them to transform into cytotoxic T-cells. Cytotoxic T-cells systematically destroy the cells in the grafted tissue. The indirect pathway is associated with chronic rejection due to the immune systems memory effects. In this case, a cell from the donor is engulfed by a recipient immune cell, which will subsequently present the donor MHC peptide on its surface. T-cells interact with this peptide and transform into helper T-cells, which secrete cytokines. These cytokines act as signals to induce the proliferation of B-cells into plasma cells, which ultimately produce antibodies. These antibodies mark the MCH antigens on the donor organs cells for destruction. These processes lead to inflammation resulting in scarring and eventual loss of function of the organ. Therefore, individuals who have received organ transplants are required to be on a lifelong course of immunosuppressant drugs that have their own set of potential problems including loss of ability to fight pathogens and even cancer cells.
The Good News!
As the need for transplantable organs has grown, so has the field of tissue engineering. Researchers have been exploring methods of generating patient specific human tissues in the laboratory with the hope of one day eliminating the long waitlists and the prospect of graft rejection. However, despite its compelling implications, in-vitro tissues have not proven to be an ideal alternative for natural human organs for a fundamental reason. Organs are composed of cells living in an extracellular matrix (ECM) that have been infiltrated with a complex network of blood vessels. One of the biggest challenges of generating functional organs in a laboratory is to ensure that they have the fundamental architecture to ensure proper blood perfusion to the organ’s most remote regions. Improper oxygen and nutrient delivery to the organ’s cells puts the survival of the graft in jeopardy. This is particularly challenging for thick dense organs such as livers and kidneys. Therefore, instead of attempting to put an organ together ourselves, why don’t we start with one the way Mother Nature intended?
Decell/Recell Technology
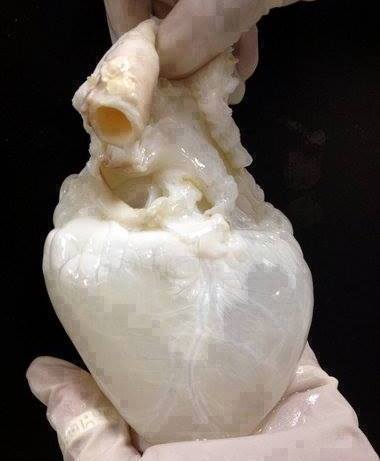
Decellularized Heart. By: 2il org, Permission: CC BY 2.0. Not Modified
Perfusion decellularization or decell/recell is a technology first demonstrated in 2008 in which cells are removed from a whole organ while maintaining the ECM scaffold with its original mechanical properties and vascular networks. This technique uses the organs native vasculature to perfuse a detergent throughout the entire organ. Previously, immersion decellularization was the primary technique used to decellularize tissues. This is a process in which the organ is immersed in a detergent while mechanical and enzymatic processes are employed to remove cells from the EMC. Decell/recell is ultimately more effective because it increases the surface area of the detergent and decreases time requires for cellular removal. A whole organ can be decellularized in as little as one day by removing the cells from inside out! More importantly, since mechanical and enzymatic methods are not used the capsule of the organ is preserved, making it capable of maintaining physiological pressures.
However, this is where it gets interesting. After the old cells of the organ have been removed, organ can be seeded with the patient’s own stem cells. Once these stem cells have been injected, they migrate to their proper microenvironments and commence differentiation as the cells grow and mature in a bioreactor. Due to the fact that the patient’s own cells are used eliminates the need for pharmacological immunosuppression and graft rejection after the transplant.
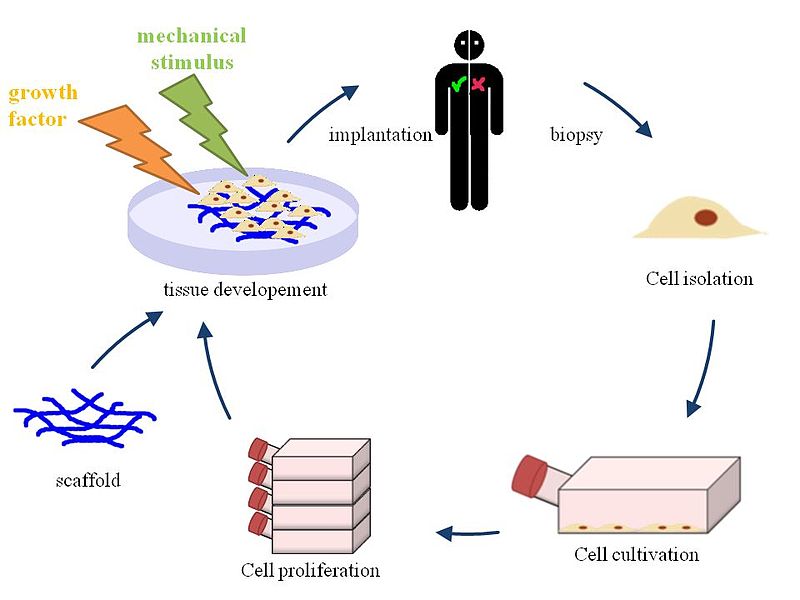
Tissue Engineering. By: HIA, Permission: CC BY 3.0. Not Modified
There is no question that this technique is one of the most exciting breakthroughs in regenerative medicine to date and may one day be a viable cure for end-stage organ failure. It has been shown that in animal tissues, that the decell/recell method is important for ensuring appropriate blood perfusion to the organ and that the ECM effectively allows stem cells to migrate and establish themselves to their appropriate locations. In addition, these cells have been shown to start completing their specific functions and have been successfully transplanted into animal models, but not to the same extent as natural organs. However, we will not be eliminating the need for organ transplant wait lists anytime soon. Even though progress has been made with this technology it is important to understand that this is not a trivial task and researchers are still face with challenges, including identifying appropriate cell sources, recellularizing with appropriate cell numbers and maintaining organs in vitro. Therefore, a lot of progress needs to be made before the can be used on actual patients. However, with this a technology a world without long transplant wait lists and invasive immunosuppressive drug regimes is within reach.

Recent Comments