We are used to forgetting things from time to time, perhaps more often when we are stressed or tired. We forget trivial things such as where we last left our keys, or to pick up bananas from the grocery store, or even appointments we have; we are not however, used to forgetting who our loved ones are or something as basic as language for communication. When someone is diagnosed with Alzheimer’s, daily living becomes progressively more difficult as the disease advances.
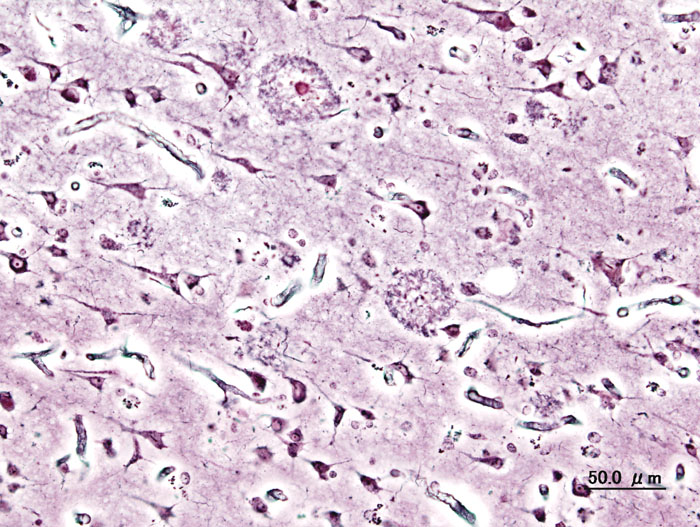
Neuritic plaques present in AD brain By User:KGH – User:KGH, CC BY-SA 3.0, https://commons.wikimedia.org/w/index.php?curid=552916
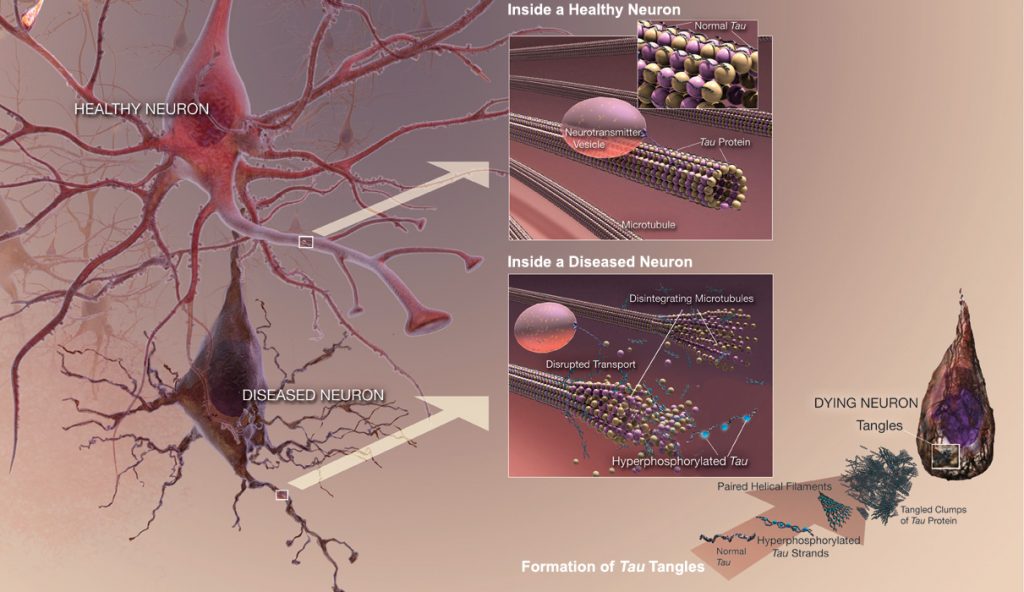
Abnormally phosphorylated tau filaments in AD brain By National Institute on Aging – http://www.nia.nih.gov/alzheimers/publication/alzheimers-disease-unraveling-mystery, Public Domain, https://commons.wikimedia.org/w/index.php?curid=25136530
Alzheimer’s disease (AD) is the most common form of dementia, accounting for 60-80% of all dementia cases (Pan et al. 2016). It is a fatal neurodegenerative disorder, with a complex pathogenesis involving numerous factors, all of which are not yet known (Pan et al. 2016). Clinically, it is characterized by progressive memory loss, mood changes, difficulties in communication and reasoning, ultimately ending in the loss of independent living; pathologically it is characterized by the accumulation of extracellular β-amyloid (Aβ) and abnormally phosphorylated tau filaments in neurons, leading to neuritic plaques and neurofibrillary tangles, respectively (Pan et al. 2016). Several markers of inflammation have been identified in the brains of AD patients, as well as microglia localized in the vicinity of Aβ plaques in the brain (ElAli & Rivest 2015). Microglia are mononuclear phagocytes that comprise the main immune cell population within the brain, constituting up to 10% of cells in the brain (ElAli & Rivest 2015). These cells are derived from myeloid precursors that penetrate into the brain during the various developmental stages, with generation of 95% of microglia occurring postnatally when the formation of the blood-brain barrier (BBB) is complete (ElAli & Rivest 2015).
Formation of β-amyloid plaque By National Institute on Aging – http://nihseniorhealth.gov/alzheimersdisease/whatisalzheimersdisease/01.html, Public Domain, https://commons.wikimedia.org/w/index.php?curid=25038029
Microglia support and regulate neuronal function in healthy brains. Unlike many other cells present in the brain, microglia are dynamic in nature and undergo remodelling processes throughout the lifespan (ElAli & Rivest 2015). Microglia are the resident macrophages of the central nervous system (CNS) constantly surveying the brain environment, while phagocytizing apoptotic cells in regions of neurogenesis and in synapse removal during development and shaping synapses in the adult brain, thus essential in brain homeostasis (ElAli & Rivest 2015). These cells are also the dominant antigen-presenting cells, and through interaction with T lymphocytes, mediate the immune response in the brain during injury. Elimination of apoptotic cell debris via phagocytosis prevents the build up of excessive accumulation of cell debris within the brain that would otherwise result in a destructive inflammatory response (ElAli & Rivest 2015). The phagocytic properties of microglia are modulated by both pro- and anti-inflammatory cytokines and are greatly affected by physiological aging, epigenetics, and genetic factors (ElAli & Rivest 2015). The degradation of internalized targets of microglia is at least partially characterized by activation of respiratory burst, namely the intracellular release of reactive oxygen species (ROS), and an inflammatory response (ElAli & Rivest 2015). Microglia are the exclusive source of ROS, which are suggested to participate in the degradation of targets inside phagosomes; however, phagocytes are not always able to process the internalized targets in phagosomes properly, leading to the release of toxic molecules, including ROS, a phenomenon termed frustrated phagocytosis (ElAli & Rivest 2015).
In AD, microglia present changes in phenotype and morphology, corresponding to an activated state (Krabbe et al. 2013). The microglia are attracted to the Aβ plaques present in AD brains and are involved in their clearance directly through phagocytosis or indirectly through production of enzymes of Aβ degradation (ElAli & Rivest 2015). Microglia will also produce elevated levels of proinflammatory cytokines as well as reactive oxygen species when activated (Krabbe et al. 2013). The proinflammatory molecules that are released when activated with proteins from Aβ include nitric oxide and tumor necrosis factor alpha (TNFα) (Krabbe et al. 2013). The interaction between microglia and Aβ plaques is highly proportional, such that larger plaques are surrounded by larger microglia and any change in size of the plaques are tightly associated with a similar change in microglial volume (ElAli & Rivest 2015).
This all sounds like microglia are the heroes in protecting the brain during times of injury and thus the answer behind a cure for Alzheimer’s. Why then, does Alzheimer’s disease progress while microglia are hard at work? As the disease progresses and more Aβ plaques accumulate, microglia become less efficient in removal and degradation, consequently becoming dysfunctional as the burden of plaque overwhelms the cells (Krabbe et al. 2013). In addition to the burden of plaques, the chronic production of inflammatory molecules and the persistent exposure to a pro-inflammatory microenvironment within AD brains influences microglial function negatively (Krabbe et al. 2013). Early activation of microglia is beneficial in clearing the toxic Aβ plaque accumulation, but too much of one thing never turns out to be good. The sustained activation of microglia leads down a detrimental path by exacerbating the inflammation, increasing Aβ deposition, as well as accentuating the neurodegenerative cascade (ElAli & Rivest 2015). Production of pro-inflammatory cytokines and neurotoxic molecules through frustrated phagocytosis contributes to this exacerbation. The stimulation with pro-inflammatory cytokine TNFα leads to the downregulation of receptors involved in Aβ binding and phagocytosis, subsequently reducing the removal of Aβ by phagocytosis (Krabbe et al. 2013). Neuronal injury thus results from the uncontrolled activity of microglia within the brain, contrasting its initial beneficial role.
There is no known cure for Alzheimer’s disease, and no way to stop it from developing. Alzheimer’s is a disease that instils fear in the back of everyone’s mind, as we cannot imagine forgetting the wonderful life we have lived and the people we love the most. At this point in time, we are not able to console the depth of fear felt towards the disease and towards those who are facing it everyday. Microglia function in the Alzheimer’s brain may be a direction to take in discovering the underlying causes of the disease and developing a cure. As knowledge of the pathophysiology in the Alzheimer’s brain broadens, the beneficial and detrimental activities of microglia can be fully understood.
Recent Comments