Aging. Something we seem to revel in during the tender golden years of youth and regret once we pass the 45-year-old achievement, if not earlier. Advances in healthcare, new medical procedures, and increased awareness of factors affecting our standards of living have led to increased longevity rates worldwide. The United Nations estimates the worldwide proportion of people 65 and older to increase by 188% from 2010-2050, while the proportion of people over the age of 100 is projected to increase by 1004%. How is that for a stat!
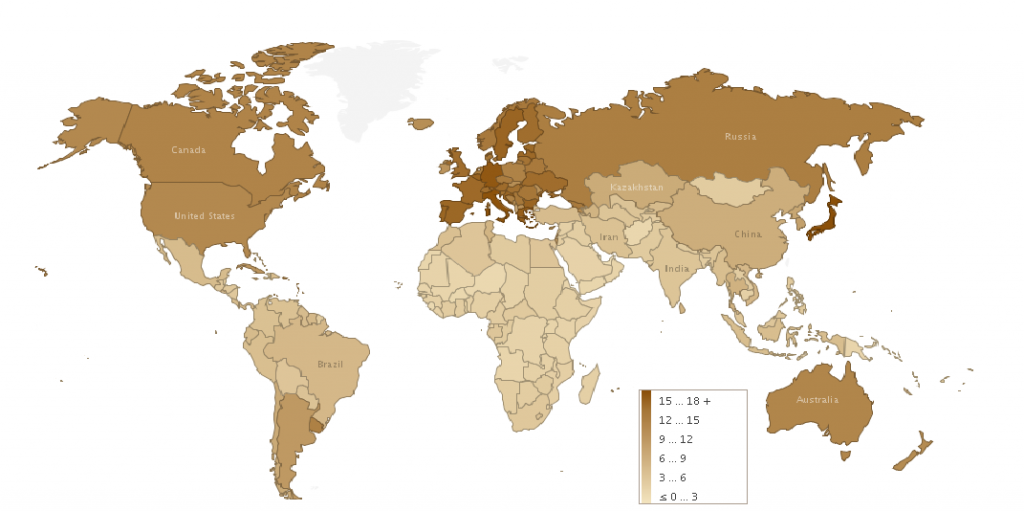
UN’s 2005 estimate of the percentage of people above 65 years old per country. (Licensed under the Creative Commons Attribution 3.0 Unported) Source: https://commons.wikimedia.org/wiki/File:Percentage_of_Population_Above_65_-_2005.png
Accompanying our increased lifespans is the transition of leading causes of death from parasitic and contagious diseases to chronic diseases. Chronic diseases, which are most prevalent in elderly people, now place heavy burdens on healthcare systems worldwide. They often require long-term monitoring and surveillance, expensive and complex techniques and procedures, and unfortunately many do not yet have cures. Rather, treatment focuses on attempting to minimize pain, prolong life, and maintain as high a quality of life as possible in patients. One of these chronic diseases is Rheumatoid Arthritis (RA).
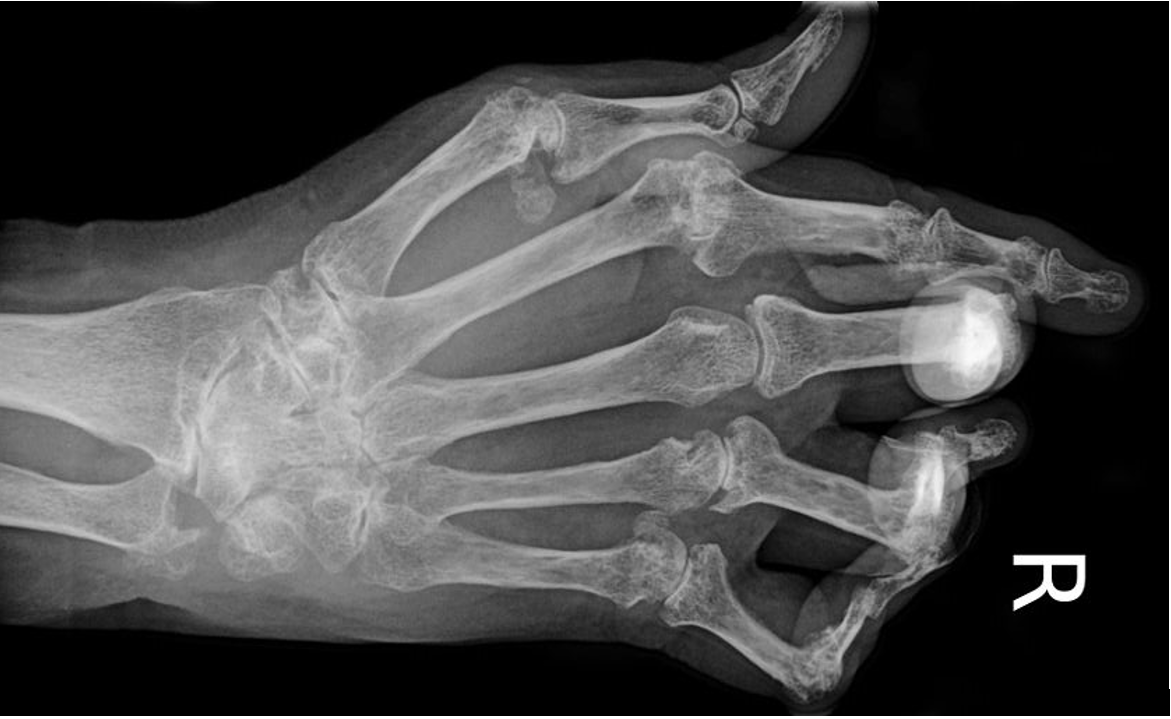
RA is one of the most common forms of autoimmune arthritis, and onset is most common in people aged 40-60 years old . In 2011, the Arthritis Alliance of Canada predicted that around 272,000 people in Canada were living with RA; a number that does not account for undiagnosed cases. If left untreated, around 50% of people with RA become unable to work due to disability and joint impairment. It commonly entails impaired synovial joint mobility as well as joint inflammation, swelling, stiffness, and pain. It is a progressive disease that damages joints and can lead to irreversible joint damage, so early treatment and intervention are key. It results from dysregulated adaptive immunity, in which cells such as T cells, B cells, and macrophages improperly recognize the synovial lining of joints as foreign, leading to an immune response. RA is important to me as my mother is afflicted by the disease, and I have seen the tremendous impact it can have on one’s life. My mother is an exceptionally active individual but RA makes some basic exercises and movements painful for her, which makes the disease all the more heart-wrenching. It certainly makes me appreciate things I sometimes take for granted, such as being able to hold a pen without clenching my teeth in pain or getting dressed in the morning without agony.
Source: http://www.niams.nih.gov/hi/topics/arthritis/rahandout.htm
Biological therapies have focused on identifying cells and cell products involved in RA, leading to positive outcomes such as decreasing inflammation levels by using cytokine antagonists to inhibit interleukins and tumour necrosis factor. A cell type shown to be intricately involved in RA are fibroblast-like synoviocytes (FLS).
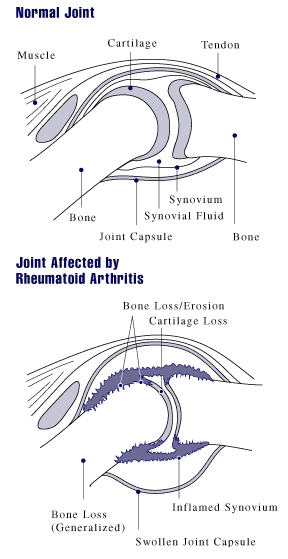
FLS are involved in producing local cytokines, inflammatory mediators, and proteolytic enzymes that degrade the extracellular matrix . In RA, FLS proliferate in number and size and also develop increased resistance to apoptosis leading to the aggravation of joint pain and inflammation. Bone and cartilage are also broken down by FLS via proteases and proteolytic enzymes. FLS are commonly found in the intima lining of the synovium encapsulating synovial joints. The intima lining produces synovial fluid to lubricate the articulating surfaces of bones and so is found on the interior of synovial joints. It is normally 2-3 cells thick and is porous to supply nutrients to avascular cartilage in the joint. In RA, the synovium becomes inflamed and is eventually overrun with immune cells including T lymphocytes and CD8+ T cells. Blood vessels grow and the intima lining can become up to 10-20 cells thick. The formation of a pannus may also occur, which acts like an invasive tumour and consists of FLS and osteoclasts. Due to the large role of FLS in producing some of the symptoms of RA, it has become the target of research aimed at countering or minimizing the joint erosion of RA.
In a study by Huang et al. in 2016, they looked at the capabilities of niclosamide on inducing apoptosis in FLS of synovial tissue samples from RA patients. Niclosamide has been shown to have anti-cancer and anti-inflammatory properties, as well as the ability to inhibit tumour growth. It can also produce reactive oxygen species (my o chem prof would be so proud!), promoting apoptosis via oxidative damage as well as damaging DNA. The authors exposed the synovial samples to different concentrations of niclosamide for different time periods, and then analyzed cell viability by MTT assay . Their results indicated a significant association between niclosamide concentration/exposure time to decreased viability and increased apoptosis of RA FLS. However, the viability of FLS in non-RA tissue was not affected. The authors indicated this could represent a selective cytotoxicity of niclosamide for FLS in RA tissue, which would greatly benefit potential treatment plans. In addition, niclosamide decreased mitochondrial membrane potential leading to mitochondrial apoptosis and the release of cytochrome C from mitochondria, which is an intermediate in the intracellular apoptotic pathway. Niclosamide also effectively up-regulated Bax expression, a proapoptotic protein in the Bcl-2 family.
These results could lead to niclosamide potentially being used as a therapeutic agent for patients with RA by extrinsically apoptosing FLS. This is especially relevant to RA due to the increased resistance of FLS to apoptosis in RA-afflicted joints. By decreasing FLS levels, cartilage and bone destruction could be minimized or even prevented, and the inflammation of the intima lining of affected synovial joints could be effectively countered. Such a treatment would not be a cure for patients with RA due to the multifactorial nature of the disease, but could possibly help them maintain mobility for longer periods of time and reduce inflammation levels. Consequently, leading to a higher standard of living and better prognoses for patients.
I find it fascinating how research can uncover the intricate mechanisms behind diseases and use it to the advantage of patients. A single mutation in a gene or a misfolded protein can have profound effects on our health, illuminating the importance of every factor in a cellular process. The more I learn about cells, the more I am amazed about how they are able to properly function considering all the possible mechanisms for error.
Recent Comments