The Curious Case of Benjamin Button, a 2008 movie starring Brad Pitt, follows the story of a man who ages backwards. The plot, supposedly inspired by Progeria, sparked interest in both popular culture and the medical community. That being said, this is not the only reference to a premature aging disorder in popular culture. Progeria has been alluded to in the media for decades; making appearances in Charles Dicken’s Bleak House and even an episode of Star: Trek Voyager! But what exactly is the science behind progeria?
Overview of Hutchinson-Gilford Progeria Syndrome
Hutchinson-Gilford Progeria Syndrome (HGPS) is an extremely rare premature aging disorder that currently affects approximately 350 children world-wide. This syndrome is characterized by the prevalence of classical aging hallmarks at a young age which include lack of subcutaneous fat, hair loss, cardiovascular disease, and death due to heart attacks and strokes in children.
HGPS is caused by a point mutation in the LMNA gene. This gene codes for the lamin A and lamin C proteins. The HGPS mutation creates a mutated isoform of lamin A, referred to as the progerin protein. Progerin has a cryptic splice site, rather than a critical cleavage site. This mutated protein associates with the nuclear membrane, interfering with normal lamina function and triggering dramatic changes in nuclear morphology. This prevents the movement of lamin proteins in the nucleus of affected cells, creating a thicker and stiffer lamina. In addition to this, HGPS patients have a notable alteration in their chromatin structure and epigenetic markers. When coupled with the thickened lamina, DNA repair kinetics are slowed in affected cells, as a result of reduced recruitment of DNA damage response factors.
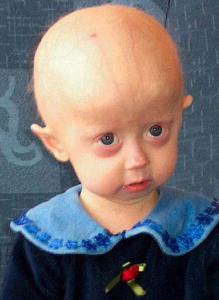
A patient with HGPS. Image Credit: Scaffidi P, Gordon L, Misteli T, CC BY 2.5
Before exploring the role of p53 in HGPS, what is the role of p53 in normal cells?
p53 is a tumor suppressor protein that prevents the development of cancer in humans. In fact, the gene coding for p53 is the most commonly mutated gene in human cancers. When a cell encounters stressors such as DNA damage, p53 responds by mediating cellular responses between apoptosis, cellular senescence, and DNA repair. These responses are coordinated with the ultimate goal of maintaining genomic integrity.
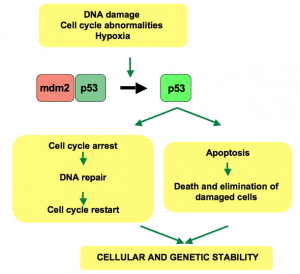
The cellular responses mediated by p53 in human cells. Image Credit: Soussi T, CC0 Public Domain
Too much of a good thing?
It would therefore appear that enhanced levels of p53 would be beneficial in human cells, right? After all, progerin has been shown to activate p53, and p53 does work to reduce the occurrence of cancer.
Interestingly enough, too much p53 has been linked to the onset of an early ageing phenotype. The mechanism by which progerin activates p53 is unknown; however, research suggests that the production of progerin leads to p53 dependent premature senescence, in amongst other clinical manifestations including genetic instability and abnormal nuclear morphology. It is important to remember that cellular senescence plays an important role in the normal aging process: as telomeres shorten, the cell enters a state of cell cycle arrest. However, in HGPS patients this survival mechanism begins to hinder more than it helps the cells.
One potential hypothesis regarding the progerin induced activation of p53 is that progerin interferes with the coordination of DNA replication factors. This causes cellular stress during replication, which signals for the activation of p53 to respond to the stress, resulting in premature cell senescence.
To analyse this relationship from a different point of view: normal human cells could be immortalized by enforced telomerase expression; however, HGPS affected cells would need to have both enforced telomerase expression and the added condition of p53 repression in order to be immortal.
Safe Interactions With Progerin
Although progerin is responsible for causing the devastating symptoms of HGPS, it is interesting to note that progerin is produced not only in HGPS patients, but also in unaffected patients. The amount of progerin in patients unaffected by HGPS is significantly lower than in HGPS affected patients. It is thought that over the life span of unaffected patients, the levels of progerin will increase, and may contribute to age related defects which are observed at an accelerated rate in HGPS patients. The main age-related defect used as a marker in the study of this phenomena is in the build-up of atherosclerosis plaques.
Conclusion and Future Directions
Much effort has been and continues to be directed towards the treatment of HGPS. The ultimate goal of this research is to decrease the levels of progerin produced, thereby mitigating the symptoms of this syndrome. So far, the pharmaceutical industry has been developing drugs that are aimed at controlling and rescuing the heterochromatin organization that is often altered in HGPS patients. One drug, called Mevinolin, acts as a farnesyl-transferase inhibitor, which prevents the addition of a critical farnesyl group in the formation of progerin. A second compound, called Trichostatin A, is being investigated, as it acts as a histone deacetylase inhibitor and may be effective in preventing chromatin rearrangement in HGPS patients.
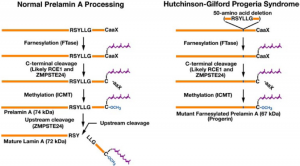
Differences between normal Prelamin A processing and HGPS Prelamin A processing. Image Credit: Coutinho HD, Falcao-Silva VS, CC BY 2.0
Advancements in the treatment of HGPS not only benefit patients of this syndrome, but also increase the scientific community’s understanding of cellular mechanisms and other physiological processes altered by rare diseases.

Recent Comments