Did you know that heart attacks are most likely to occur in the early morning? Or that completely blind people often have trouble sleeping at night, or staying awake during the day? Have you ever experienced jet-lag after you flew across time-zones? All of these phenomena have explanations based in what is called the circadian rhythm. You have probably heard of this; it’s often referred to as our ‘biological clock’, the 24-hour cycling in the physiological processes of organisms such as ourselves. This influences things like hormone levels and other physiological conditions at different times of day, in different parts of the body. But have you ever wondered how exactly our bodies maintain this biological clock?
The Biological Clock. Image Credit: Yassine Mrabet CC BY-SA 3.0.
The question can best be answered at the level of the individual cell, as it turns out.
Traditionally, the suprachiasmatic nucleus in the brain is cited as being the master control for the circadian rhythm. However, a group of researchers found that neither having a brain, nor having a body, is required to have a rhythm; individual rat cells grown in petri dishes expressed certain transcription factors independently in roughly 24-hour rhythms. Since then, most cells in the body have been discovered to have a rhythm with a complex system of feedback loops that keep it going.
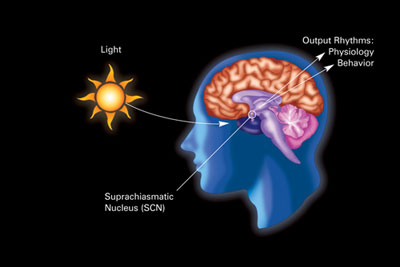
The suprachiasmatic nucleus. Image Credit: National Institute of General Medical Sciences CC0.
Here’s one simplified example of how it works: in mammals, those transcription factors include the aptly named CLOCK protein and its partner, BMAL1. CLOCK/BMAL1 start transcription of clock-related genes in the nuclei of individual cells, including the transcription of two more proteins called Period (PER) and Cryptochrome (CRY). Once made, PER/CRY inhibit and suppress the activity of CLOCK/BMAL1, thus closing off the feedback loop (it takes roughly 24 hours to cycle through). Perhaps unsurprisingly, these same cyclic proteins found in individual cells of the body are also the major players in the suprachiasmatic nucleus (video).
But what is the relevance of all this biological clock research, besides having an explanation as to why you’re unlikely to poop in the middle of the night?
As stated earlier, most cells have an internal circadian rhythm. However, like all things in life, there are exceptions; among those are embryonic stem cells, cells in the testes, and cancer cells. What’s the common factor in all of these?
A group of researchers found that the answer may be a protein called PASD1, which seems to stop the clock from functioning. They suggest that a lack of ‘clock’ in embryonic stem cells could be due to the fact that the specifics of a cell’s rhythm are very different from cell to cell, so there’s no point in establishing one until it’s been differentiated enough. This could be the same for cells in the testes, where there are more stem cell precursors than mature sperm. In cancer cells, it’s likely that a lack of clock might be what allows tumours to grow so fast. The cells aren’t limited by time of day to divide. In fact, inducing circadian rhythm in cancer cells slows cancer growth. This finding might just lead to a new way of treating cancer in the future!
A disrupted circadian rhythm also has a hand in a whole host of other metabolic diseases. Take, for example, diabetes. As you may know, the pancreas is an organ which, among other functions, helps to regulate blood sugar. There are insulin-secreting cells, which lower blood glucose levels, and glucagon-secreting cells, which raise blood glucose levels. In a healthy person, the rhythms of these two types of cells are aligned just so in order to fine-tune their secretion and maintain daily balance; but in someone who, say, works a lot of night shifts, these rhythms become disrupted. The loss of homeostasis can quickly lead to diabetes or obesity.
Another problem that can arise is heart disease. Circadian rhythm regulates blood pressure throughout the day, as seen above. Studies have shown that, yes, there are clock genes in cells of the heart; a protein called Klf15 is part of the cascade that produces the electrical current that gets the heart pumping. When these genes are removed from mice, there is more of a chance for abnormal heart rhythms, and atherosclerosis.
Ever felt a little sad during winter, when the days are short and it gets dark all too early? First of all, that’s called Seasonal Affective Disorder (SAD).
Second of all, it’s thought that those winter blues can be partially attributed to circadian rhythm disturbances: Light entering our eyes sends a signal to neurons in the suprachiasmatic nucleus, which leads to the pattern of release of neurotransmitters such as serotonin, dopamine and norepinephrine, all of which can affect mood.

What you probably shouldn’t be doing at night. Image credit: Andres Nieto Porras CC BY-SA 2.0.
When there’s not enough light, it leads to abnormalities in neurotransmitter release that can lead to depression. Conversely, if you’re on your phone in the middle of the night, the bright light shining from your screen might be messing with your body’s melatonin secretion and making it hard for you to sleep.
In conclusion, it’s clear that circadian rhythms are critical to maintaining health. A disruption of this balance can lead to some pretty nasty metabolic diseases and disorders. The bright side is that we’re beginning to become more aware of these problems, down to the level of the cell; and ironically enough people are working around the clock to find more effective solutions to combat them. We’ll need them— In today’s society, our circadian rhythms are more out of whack than ever, with the rise of industry, late hours working on the computer, and the fact that someone’s got to work the night shift. Gone are the days when humans rose with the sun. Now we rise when the alarm blares.
Of course, this doesn’t mean you’re doomed to die of an early morning heart attack in the near future, or spontaneously develop diabetes. There are a lot more factors involved. But you might be able to reduce your future risk by simply putting away your phone twenty minutes before bed tonight. The little things matter, and they add up. One change at a time.

Recent Comments