Researchers at Harvard Medical School have started trying to reverse aging in mice, and it’s working! A cool discovery in mice back in 2013 lead to a series of investigations, and implementation of an easily-administered treatment using a well-known compound already present in the body and known for the major role it plays in metabolism. “The cells of the old mice were indistinguishable from the young mice after just one week of treatment,” said the lead author of the study David Sinclair. “This is the closest we are to a safe and effective anti-ageing drug that’s perhaps only three to five years away from being on the market, if the trials go well.”
Before getting into the relatively simple treatment, let’s talk about DNA damage, which has been shown in the past to be closely associated with aging. DNA gets damaged over time, both exogenously and endogenously. Exogenous damage comes from things like exposure to radiation, smoking, and inhalation of emissions from burned fuel. To some extent, this DNA damage is controllable. If you’re careful, you can avoid the sources or at least limit your exposure (wear sunscreen, don’t smoke, etc). Endogenous damage is less easy as it tends to be spontaneous and occurs regardless of any precautions you might take. Approximately 100 000 DNA damages are expected to occur per day in the human body. This is still only ~0.03% of your body’s DNA, so not super significant. However, over time, as these mutations build up, you can have a significant problem on your hands. That’s why the cell’s DNA repair system is absolutely necessary and should be in prime condition. Without it, your DNA accumulates mutations, which impacts cell functionality and cell division. This, according to the DNA Damage Theory of Aging, is why we age and/or get age-related diseases.
The ageing of a human hand. By CC-PD-Mark [https://commons.wikimedia.org/wiki/File:Hand_aging.jpg#filelinks], via Wikimedia Commons
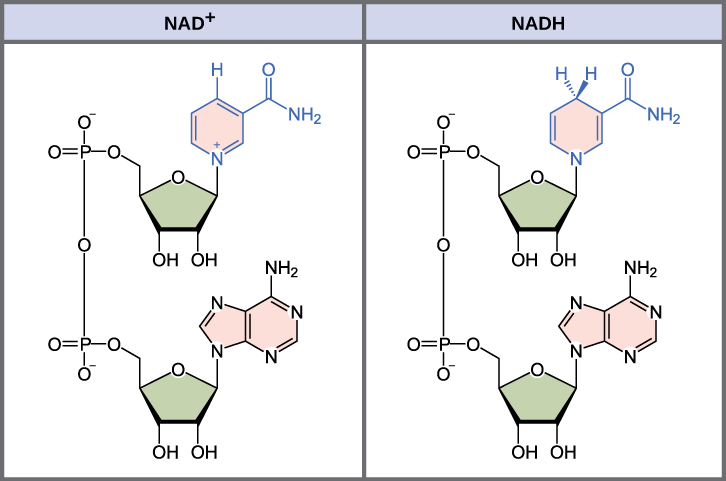
The oxidized and reduced forms of nicotinamide adenine dinucleotide. By CNX OpenStax [CC BY 4.0 (http://creativecommons.org/licenses/by/4.0)], via Wikimedia Commons
This effect is believed to be due to NAD+’s close link to DNA repair. PARP1 is one of a class of DNA repair molecules that initiates various forms of DNA repair, by ribosylating ADP. PARP unfortunately has a series of inhibitors like DCB1 (deleted in breast cancer 1) which can bind to it and limit its activity. DCB1 itself can be inhibited, however, through binding to NAD+. Low NAD+ levels leaves PARP1 vulnerable to inhibition, therefore stopping DNA repair and increasing aging. This mechanism has also been investigated in regards to cancer treatments. A series of PARP inhibitors have been introduced for the express purpose of preventing tumour cells from repairing their DNA. So far, the research is promising, though it has only really been looked at in terms of the BRCA genes, other DNA repair genes, mutations in which heavily increase the risk of female breast and ovarian cancers. The team is also collaborating with NASA to research whether NMN could possibly be given to astronauts to protect against radiation in space on a four-year journey to Mars, radiation if unchecked can lead to brain damage and weakened immune function and cancer.
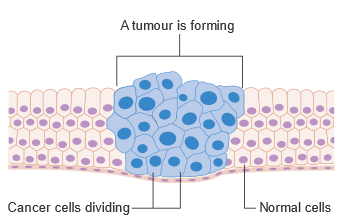
A tumour forming amongst normally functioning cells. By Cancer Research UK (Original email from CRUK) [CC BY-SA 4.0 (https://creativecommons.org/licenses/by-sa/4.0)], via Wikimedia Commons


Recent Comments